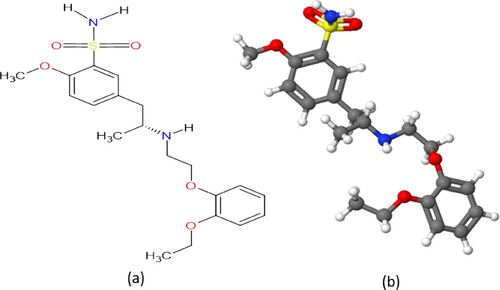
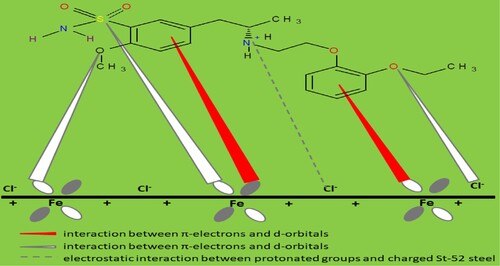
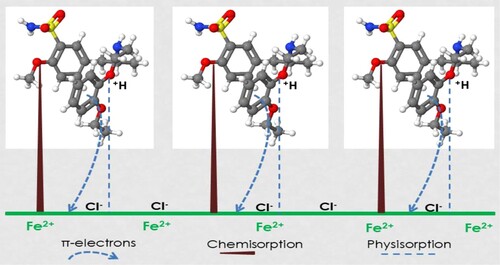
Figures & data
Table 1. Comparison of the inhibitive performance of TAM with other reported drug corrosion inhibitors.
Figure 2. Variation of (a) and (b)
of TAM with inhibitor concentration in 1 M HCl at various temperatures.
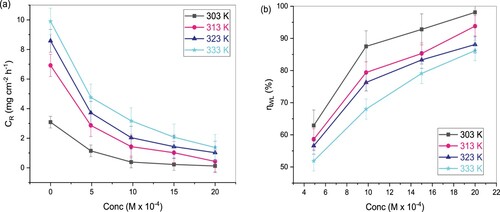
Figure 3. Plot of versus
for St52 steel corrosion in 1M HCl and inhibited solutions at various concentrations of TAM.
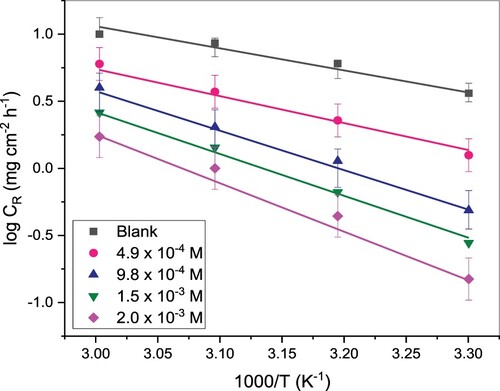
Figure 4. Plot of versus
for corrosion of St52 steel in 1 M HCl and solution and inhibited solutions at different concentrations of TAM.
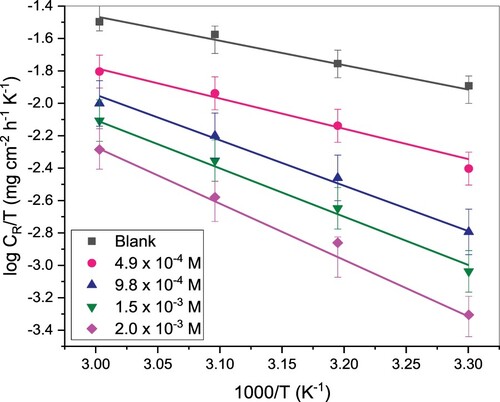
Table 2. Computed values and thermodynamics parameters for St52 steel corrosion in 1 M HCl solution without and with various TAM concentrations.
Figure 5. (a) Nyquist, (b) phase angle and (c) Bode modulus plots of St52 steel in 1M HCl without and in different concentrations of TAM.
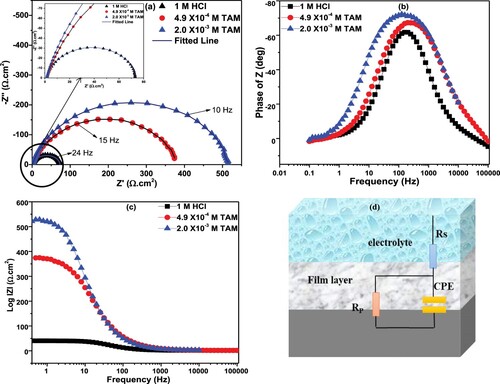
Table 3. Impedance data for St52 steel in 1M HCl and with various concentrations TAM at 303 K.
Figure 6. (a) Open circuit potential and (b) polarization curves of St52 steel in1 M HCl and in the presence of different TAM concentrations.
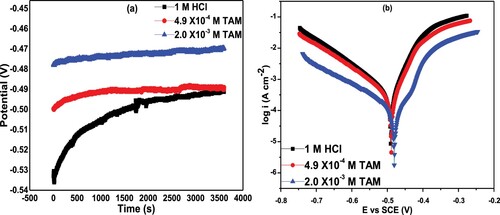
Figure 7. SEM/EDX images of St52 steel surface in (a) 1 M HCl without and (b) with 2.0 × 10−3 M of TAM.
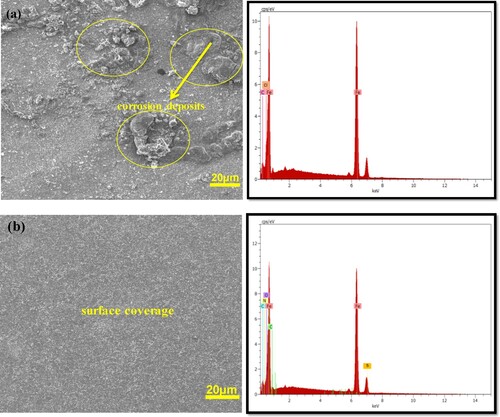
Table 4. Polarization data for St52 steel corrosion in 1.5 M HCl in the absence and presence of TAM.
Figure 8. Langmuir isotherm plots for TAM’s adsorption on St52 steel surface at various temperatures.
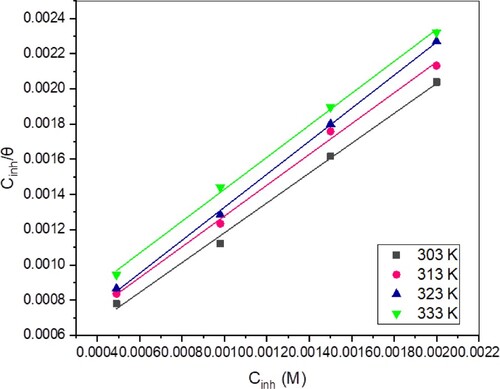
Table 5. Langmuir adsorption isotherm data for TAM at various temperatures.
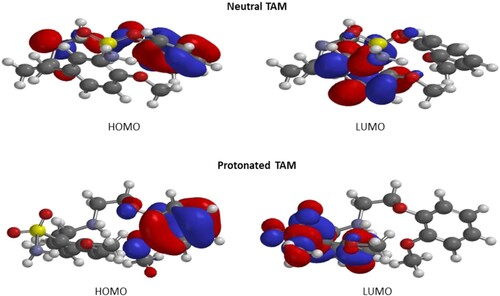
Table 6. DFT parameters for neutral (TAM) and protonated (TAM-H+) form of the inhibitor.