Figures & data
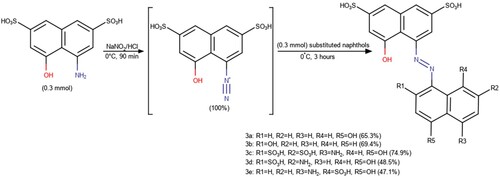
Table 1. Optimized procedures for the synthesis of 8-hydroxy-3,6-disulphonaphthyl azohydroxynaphthalenes (3a–e).
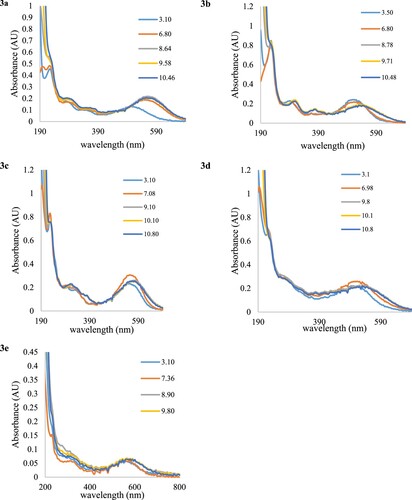
Figure 5. Electronic absorption spectra of the five dyes in (a) ethanol, (b) acetonitrile, (c) acetone, (d) ethyl acetate, (e) DMF at concentrations.
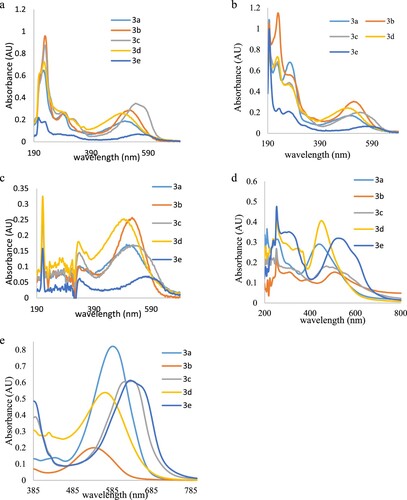
Table 2. Colours of the dyes in various solvents.
Table 3. Variation of the molar transition energies of the dyes with Kamlet–Taft parameters of solvents.
Table 4. Multiple linear regression analysis of the KAT equations for molar transition energies of the dye probes.