Figures & data
Figure 1. Wound-healing assay design Full-thickness wounds were created using a 2-mm in 6-mm biopsy punch. Photographs of “punch-in-a-punch” biopsy injury on human skin.
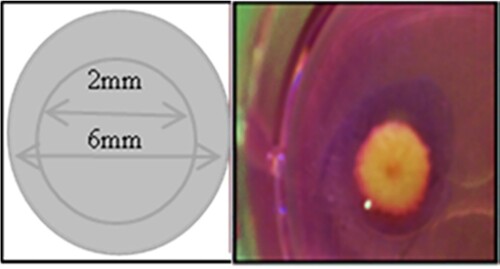
Table 1. Demographic data of participants and sources of skin explants.
Figure 2. A schematic diagram showing human wound healing biopsy measurements for determining the length of the new epithelial tongue, number of keratinocytes, and thickness of epidermis in wounded skin.
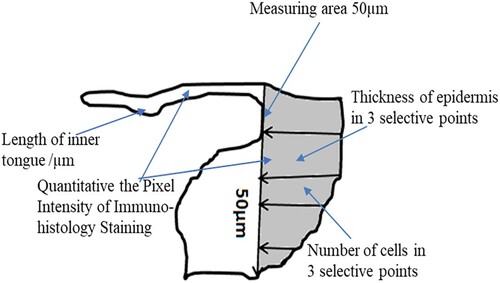
Figure 3. Re-epithelialisation of human skin in the diabetic and non-diabetic wound. The length of the new epithelial tongue in the non-diabetic section was significantly longer (14.8 μm) than that of the diabetic section (10.6 μm) after 12-day post-wounding (P = .04, n = 3). Data are illustrative of three experiments, *P < .05.
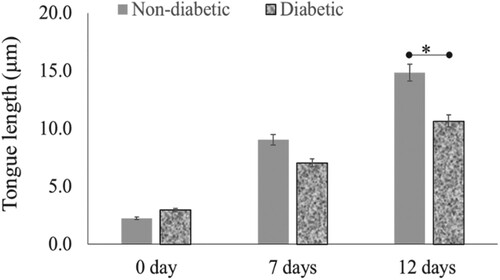
Figure 4. Re-epithelialisation of human skin wound in the absence and presence of S. epidermidis lysate. (A) H&E-stained sections of human skin wounds on day 12 (20× magnification; scale bar represents 100 μm). The new epithelial tongue is depicted by white lines. (B) Length of new epithelial tongue measured in µm at specified time periods. Significance relative to control data is denoted by *P < .05.
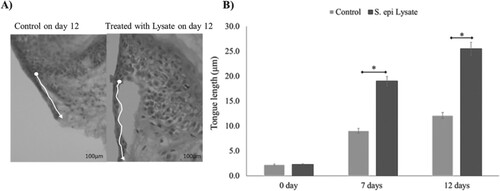
Figure 5. Effect of S. epidermidis lysate on wound re-epithelialisation rate in diabetic skin explant. (A) H&E-stained images of diabetic wound sections after treatment with or without S. epi lysate on day 12 post-wounding (white arrows indicate the tongue length at 20× magnification, scale bar represents 100 μm). (B) Quantification of wound re-epithelialisation by measuring the tongue length in diabetic patients with and without S. epi lysate after day 7 and 12 post-wounding (P = .03, n = 3). Data are illustrative of three experiments, *P < .05.
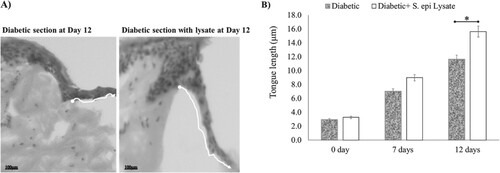
Figure 6. Effect of S. epidermidis lysate on epidermis thickness and keratinocyte numbers in human skin explant. (A) The total thickness of epidermal layers in the wound area as evaluated from H&E-stained human skin sections treated with S. epi lysate for diabetic and Non-diabetic samples (P = .03, P = .04, respectively, n = 3). (B) Cell number per unit area in the epidermis of wound area as evaluated from H&E-stained human skin sections after treatment. Significance was relative to control data indicated by *P < .05.
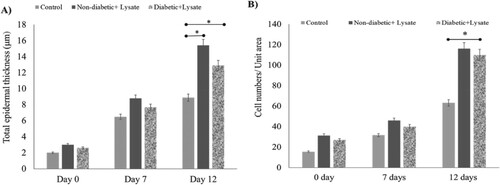
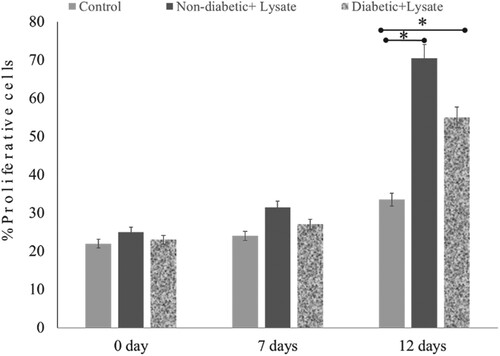
Figure 7. Effect of S. epidermidis lysate on cell proliferation in non-diabetic and diabetic human skin explants measured post-wounding. Quantification of Ki-67 immunoreactivity was done in newly formed epithelial tongues 50μm length behind the new tongue (schematic diagram 1). The number of Ki-67 positive cells was detected in the treated wound on days 7 and 12, and compared with the control (P = .02, n = 3, * P < .05).