Figures & data
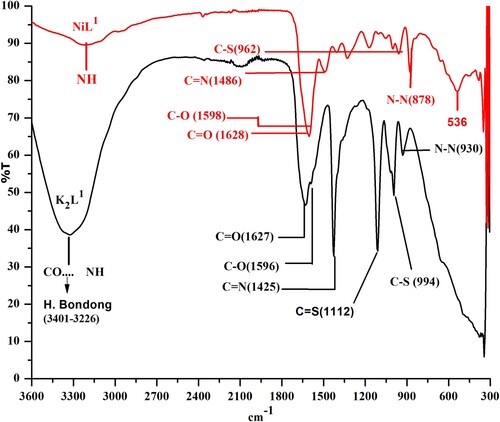
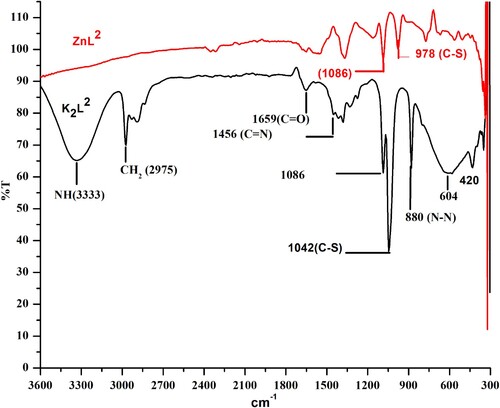
Table 1. Comparison of infrared spectral details of ligands and their complexes with the literature.
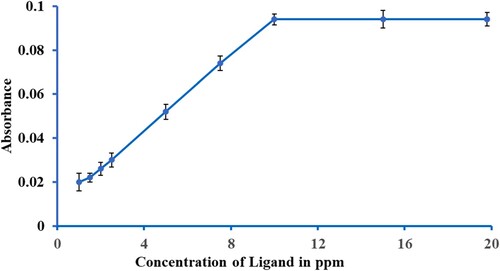
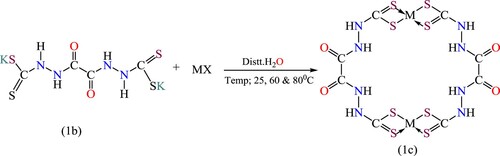
Scheme 2. The ligand-to-metal ratio in the complex formation of Ligand K2L1 (M=Fe, Co, Ni, Cu, Cd, Ag, Zn, and Pb) at various temperatures and pH (1c).
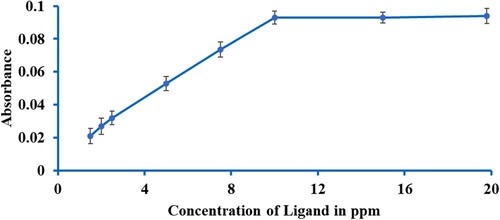
Scheme 3. The ligand-to-metal ratio in the complex formation of Ligand K2L2 (M=Fe, Co, Ni, Cu, Cd, Ag, Zn, and Pb) at various temperatures and pH (2c).
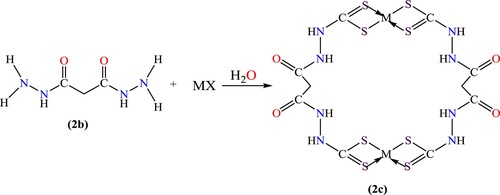
Table 2. Metal removal efficiency of dithiocarbamate ligands (K2L1-2) at various pH.
Table 3. Metal removal efficiency of dithiocarbamate ligands (K2L1-2) at various temp (°C).