Figures & data
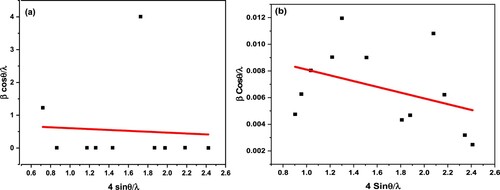
Table 1. Lattice parameters, particle, crystalline size and strain values of compound.
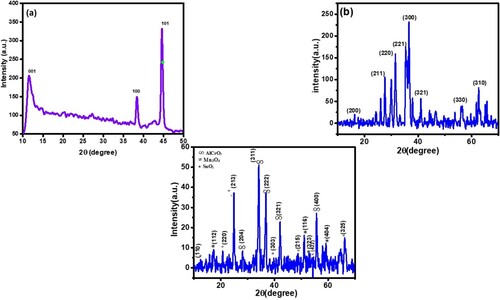
Table 2. Miller indices and peak lists of AlCrO3 and GO/AlCrO3/SiO2/Mn3O4/SnO2 nanocomposite.
Figure 5. Grains diameter variation and Histogram distribution of GO@AlCrO3@SiO2@Mn3O4@SnO2 nanocomposite.
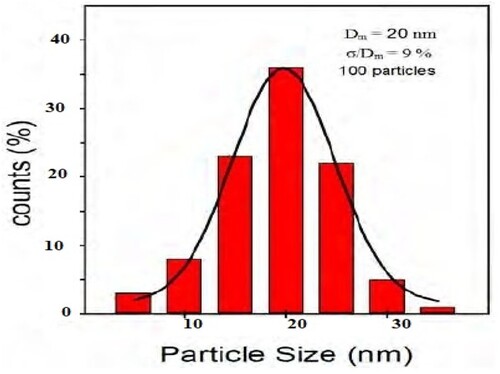
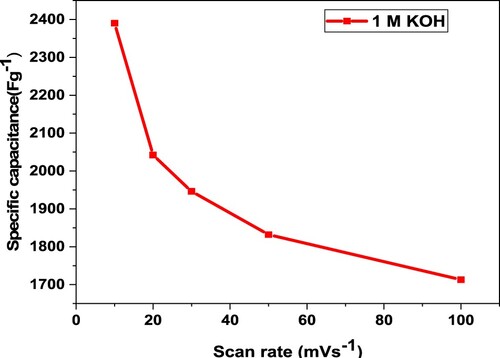
Table 3. Measurements of specific capacitance of GO@AlCrO3@SiO2@Mn3O4@SnO2 at various scan rates.
Figure 14. (a) GCD curves and Ragone plot of GO@AlCrO3@SiO2@Mn3O4@SnO2 nanocomposite in IM KOH electrolyte (b) Cycling performance.
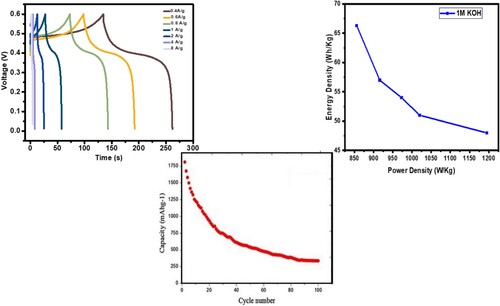
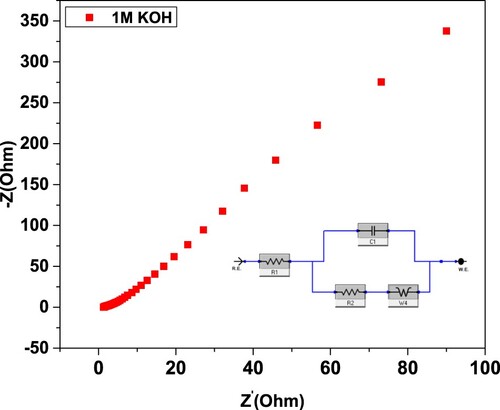
Table 4. GO@AlCrO3@SiO2@Mn3O4@SnO2 nanocomposite electrode material parameters.
Table 5. Comparison of multinary composite with previous literature.