Figures & data
Table 1. Base parameters of the solved examples (reference conditions: P = 82.425 kPa, T = 323.15 K).
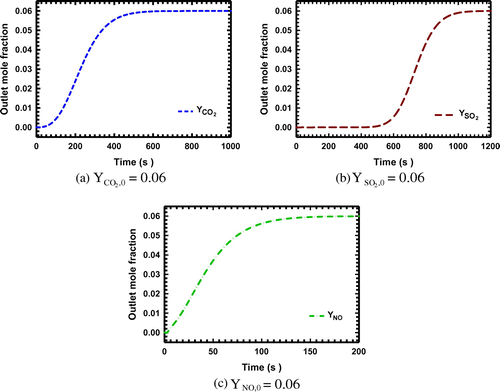
Figure 3. (a)–(d): The breakthrough curves for the system with CO2 and SO2 as the adsorbing components on zeolite NaX.
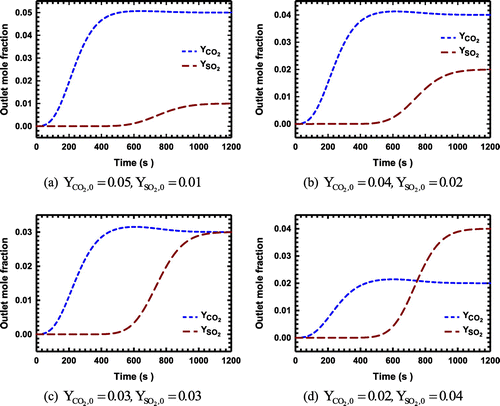
Figure 4. (a)–(d): The breakthrough curves for the system with CO2 and NO as the adsorbing components on zeolite NaX.
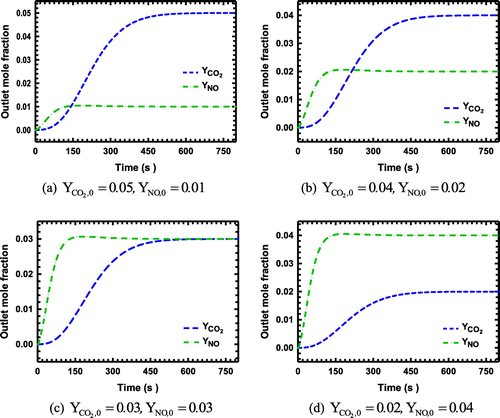
Figure 5. (a)–(d): The influence of adsorption parameters on breakthrough curve for the system with CO2 as the adsorbing component on zeolite NaX.
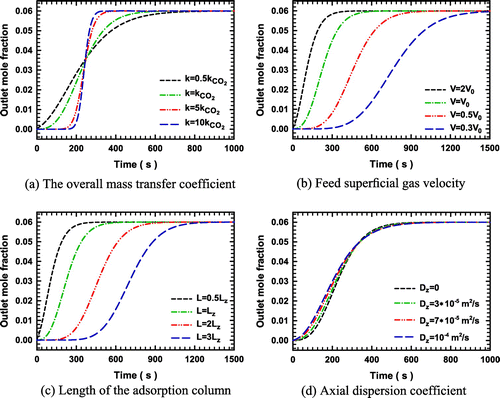
Figure 6. Effect of temperature on the breakthrough curves of CO2 adsorption on OXA-GAC (a) at a 50 mL min−1 feed and (b) at a 100 mL min−1 feed flow rate.
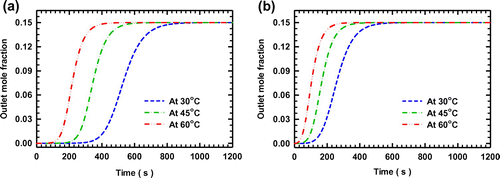
Figure 7. Comparison of the artificial experimental data and estimated values of the breakthrough curves of the system with CO2 and SO2 as the adsorbing components on zeolite NaX.
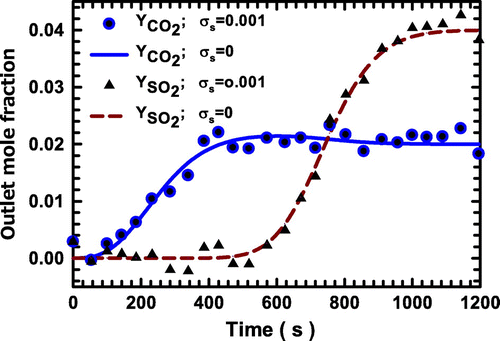
Table 2. The estimated overall mass transfer coefficients, percentage of errors, corresponding CPU times and number of iterations at different mole fractions for the system with CO2 and SO2 as the adsorbing components on zeolite NaX.
Table 3. The estimated overall mass transfer coefficients, percentage of errors, corresponding CPU times and number of iterations at different mole fractions for the system with CO2 and NO as the adsorbing components on zeolite NaX.
Table