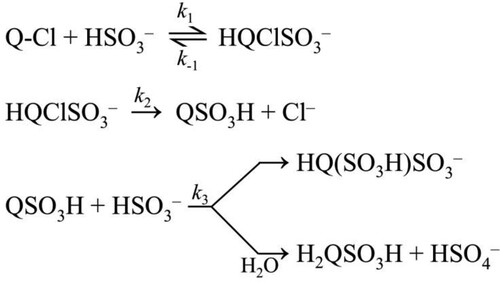
Figures & data
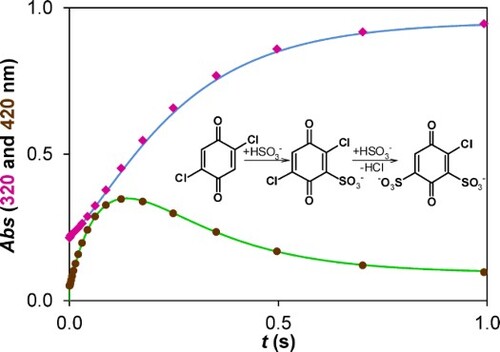
Figure 2. Spectral changes in the photochemical decomposition of 2,5-DCBQ (λmax = 525 nm) recorded by the SPECORD S600 diode array spectrophotometer. The optical shutter was kept open during this experiment, i.e. the sample was continuously illuminated by the light source of the spectrophotometer. The absorption maxima at 525 and 345 nm are consistent with the formation of hydroxy-2,5-DCBQ and 2,5-DCBQH, respectively. c(2,5-DCBQ) = 0.50 mM.
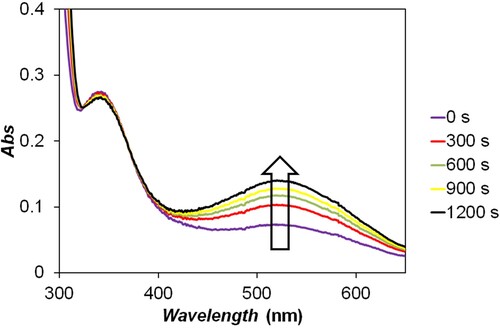
Table 1. Relative intensity values of sulfite radical anion (SO3·–), hydrogensulfite ion (HSO3–), sulfate radical anion (SO4·–) and hydrogensulfate ion (HSO4–) in the tandem mass spectra of the base peak in the RBQs – S(IV) reaction.
Table . Identified chemical formulas, proposed structures, m/z values, molecule ion types and relative intensities of the products in the reactions of CBQs and sulfur(IV) in unbuffered medium on the basis of high resolution MS experiments.
Table 3. Mass spectrometric identification information for the products in the reactions of CBQs and sulfur (IV) in unbuffered medium and data of relevant reference materials.
Figure 3. Characteristic kinetic traces for the reaction of 2,5-DCBQ with sulfur(IV) at two different wavelengths (320 and 420 nm). c(2,5-DCBQ) = 0.50 mM, c(S(IV)) = 0.15–5.00 mM (the individual concentrations are listed in the figures), pH = 3.8 (acetate buffer), I = 1.0 M, T = 298.2 K.
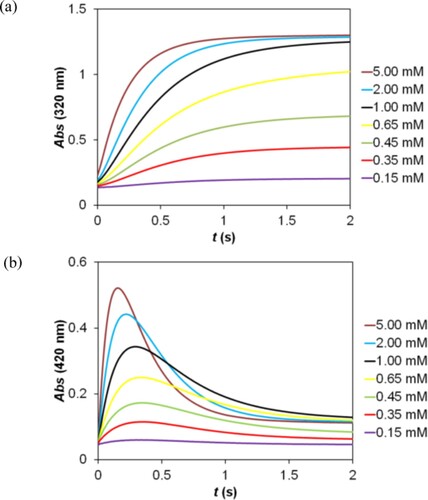
Figure 4. Kinetic traces recorded as a function of the 2,5-DCBQ concentration (a) and pH (b) in the reaction of 2,5-DCBQ with sulfur(IV). (a): c(2,5-DCBQ) = 0.015–0.50 mM, c(S(IV)) = 5.00 mM, (acetate buffer) pH = 4.5. (b): c(2,5-DCBQ) = 0.50 mM; c(S(IV)) = 5.00 mM, pH = 3.6–5.1 (acetate buffer), I = 1.0 M, T = 298.2 K.
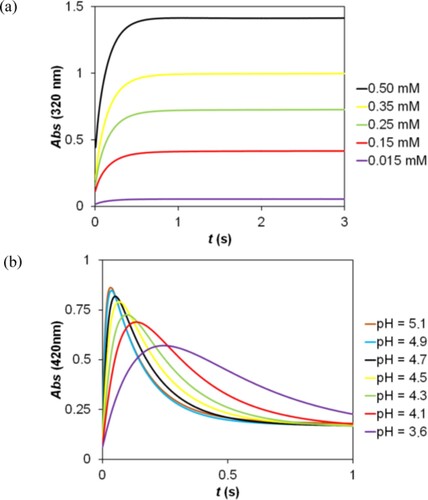
Figure 5. Determination of the stoichiometry in the reaction of 2,5-DCBQ with S(IV) by the Job method. c(2,5-DCBQ) = 0.50 mM, c(S(IV)) = 0.20–30.00 mM, pH = 3.8 (acetate buffer), I = 1.0 M, T = 298.2 K.
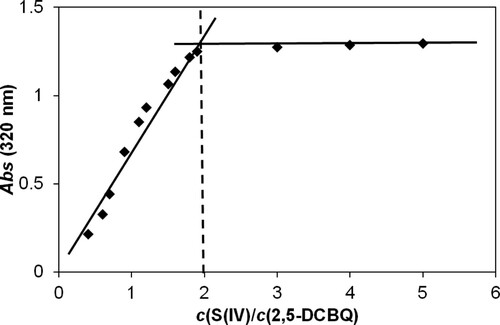
Figure 6. Experimental kinetic traces of the reaction between 2,5-DCBQ and S(IV) at 420 nm (brown circles) and 320 nm (purple rectangles), together with the fitted double exponential curves. Only a small fraction of the measured points is shown for clarity. c(2,5-DCBQ) = 0.50 mM, c(S(IV)) = 1.0 mM, pH = 4.5 (acetate buffer), I = 1.0 M, T = 298.2 K.
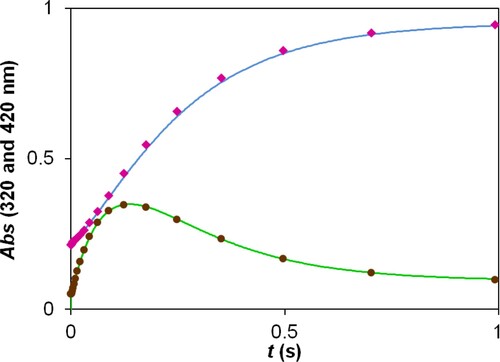
Figure 7. Experimental and fitted kinetic traces using the ZITA software. c(2,5-DCBQ) = 0.50 mM (A, B, C, D); c(S(IV)) = 10.00 mM (A, C), 2.00 mM (D), 1.00 mM (B); pH = 3.8 (A, B), 4.5 (C, D); acetate buffer; I = 1.0 M; T = 298.2 K. Only a selection (ca. 5%) of measured points is shown for clarity.
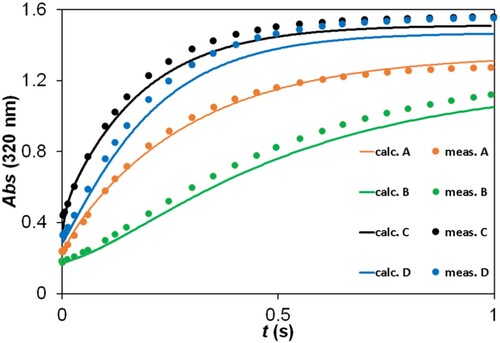
Table 4. Estimated parameters obtained by the ZITA software for the reaction of 2,5-DCBQ with sulfur(IV).