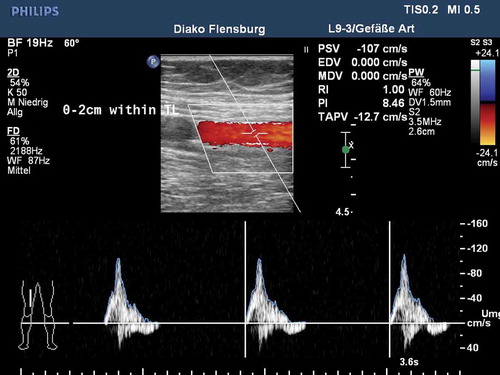
Figures & data
Figure 1. Photograph of an EluviaTM drug-eluting stent and a scanning electron microscope image of a stent strut (150x magnification). Images provided courtesy of Boston Scientific. ©2016 Boston Scientific Corporation or its affiliates. All rights reserved.
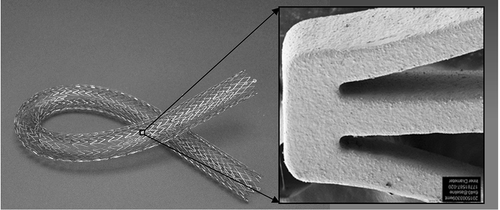
Figure 2. Paclitaxel concentration in stented swine arterial tissue. Boston Scientific data on file. Image provided courtesy of Boston Scientific. ©2016 Boston Scientific Corporation or its affiliates. All rights reserved.
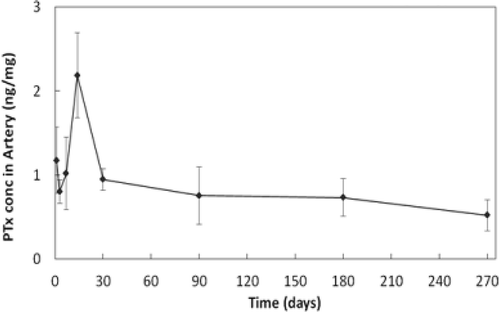
Figure 3. Kaplan-Meier estimate of primary patency at 12 months in the MAJESTIC trial. Adapted from Müller-Hülsbeck S, Keirse K, Zeller T, et al. Twelve Month Results from the MAJESTIC Trial of the EluviaTM Paclitaxel-Eluting Stent for Treatment of Obstructive Femoropopliteal Disease. J Endovasc Ther 2016, doi: 10.1177/1526602816650206.
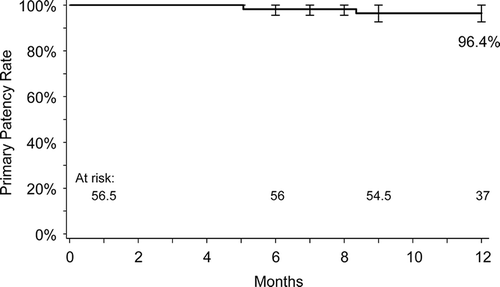
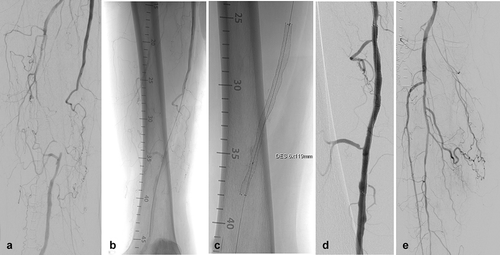
Figure 4. EluviaTM stent implantation in the MAJESTIC study. The female patient was 73 years old and a current smoker with 100% occluded lesion in the mid-SFA with severe calcification. (A) Baseline angiography of the SFA shows the occlusion of nearly 9 cm in length. (B) Unsubtracted angiography indicates an intraluminal 0.018-inch guidewire crossing. (C) Fluoroscopic view of a 6 x 119 mm EluviaTM stent. Note the vessel wall calcification located in the mid- and distal part of the successfully treated lesion. (D) Final angiography represents a good technical result without any significant residual stenosis. (E) The run-off is non-compromised and does not show any signs of distal embolization.