Figures & data
Table 1. Overview of commercial product approvals/launches based on disposable single-use pen and autoinjector device platforms. 24 out of 29 US launches of single-use disposable pre-filled syringe based autoinjectors for subcutaneous drug delivery were based on device platforms to date (as per 24 June 2019). Non-platform-based disposable autoinjector launches were: Humira® (AbbVie), Simponi® (JnJ), Avonex® (Biogen), Sumatriptan (Dr. Reddy’s), and Makena® (AMAG).
Figure 1. Illustration of the four design features for which variation was introduced.
Note: For the label color, the label without the device is shown. For the needle shield color, one device example of each color, corresponding to the small square design, is presented.

Table 2. Description of the eight autoinjector platform variants (A.1 - A.8) included in the pair-wise comparison.
Table 3. Description of the user population included in the study.
Table 4. Descriptive statistics of the pairwise similarity ratings (total sample). The similarity assessment was performed based on a nine-point Likert-type scale (1 = ‘completely similar’; 9 = ‘completely dissimilar’). The values for the mean (mea), median (med), and standard deviation (std) are reported.
Figure 2. Illustration of the two-dimensional (I) and three-dimensional (II) configuration of the self-injection device platform variants (A.1 - A.8) derived from the total sample (N = 74). The three-step coding process resulted in assigning the following device attributes to each dimension: aspect ratio (1-2D), label color (2-2D), device size (1-3D), device shape (2-3D), and device chromaticity (3-3D).
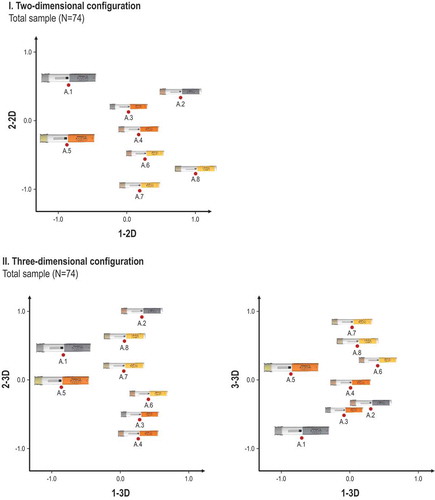
Table 5. Overview of the device attributes empirically identified as relevant for device distinguishability.
Table 6. Device attributes relevant for self-injection device variant distinguishability per user group. The results are presented for the two-dimensional (A) and the three-dimensional (B) configurations. The overall stress values indicate the goodness-of-fit and are reported separately for each user group.
