Figures & data
Figure 1. Gravitational stability of pMDI formulated as solutions or suspensions: solution-based formulations contain the drug solubilized in a hydrofluoroalkane (HFA) propellant with or without co-solvents. HFA suspensions contain suspended drug particles subjected to creaming or sedimentation according to the difference between the density of the drug and the density of the system.
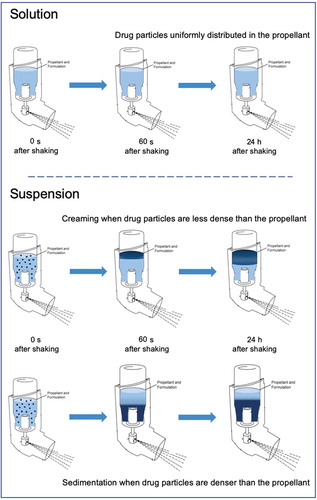
Table 1. Characteristics and therapeutic indications in European Union of fixed-dose combination (FDC_pMDIs), high dose strength fixed-dose combination (HD_FDC_pMDIs) and monocomponent pMDIs drug products.
Figure 2. Emitted dose (µg) without shaking the canister at different level of canister content for fixed-dose combination (FDC) pMDIs: Foster (BDP-FF) 100 + 6 µg, Symbicort (BUD-FF) 160 + 4.5 µg, Seretide (FP-SX) 125 + 25 µg, Trimbow (BDP-FF-GLY) 100 + 6 + 10 µg, Serzyl (FP-SX) 125 + 25 µg, Flutiform (FP-FF) 125 + 5 µg (n = 9; mean ± st.dev).
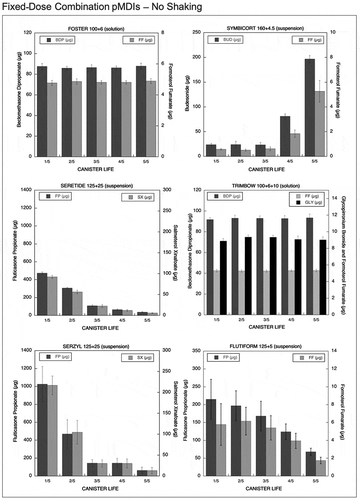
Figure 3. Emitted dose (µg) without shaking the canister at different level of canister content for high dose strength fixed-dose combination (HD_FDC) pMDIs: Foster (BDP-FF) 200 + 6 µg, Seretide (FP-SX) 250 + 25 µg, Serzyl (FP-SX) 250 + 25 µg, Flutiform (FP-FF) 250 + 10 µg (n = 9; mean ± st.dev).
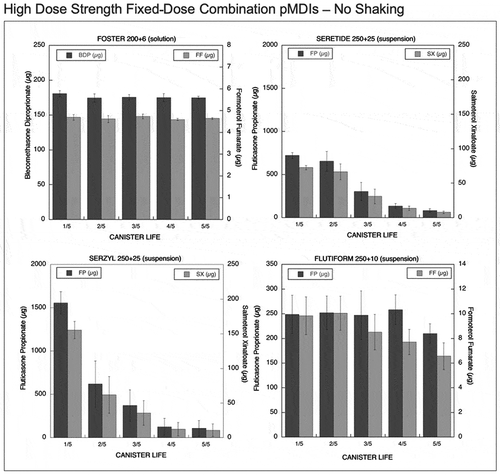
Figure 4. Emitted dose (µg) without shaking the canister at different level of canister content for monocomponent pMDIs: Clenil (100 µg BDP), Aircort (200 µg BUD), Ventolin (100 µg SS) and Flixotide (50 µg FP) (n = 9; mean ± st.dev).
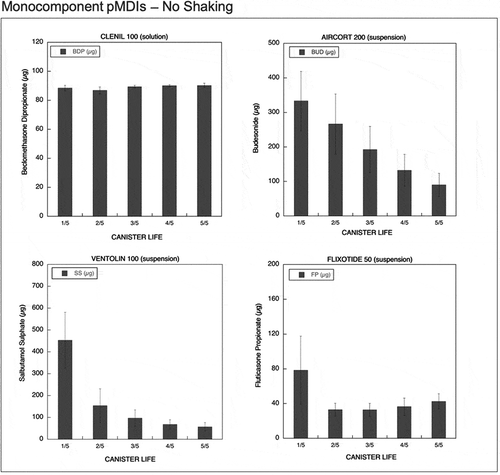
Figure 5. Emitted dose (µg) applying increasing time delays after shaking for fixed-dose combination (FDC) pMDIs: Foster (BDP-FF) 100 + 6 µg, Symbicort (BUD-FF) 160 + 4.5 µg, Seretide (FP-SX) 125 + 25 µg, Trimbow (BDP-FF-GLY) 100 + 6 + 10 µg, Serzyl (FP-SX) 125 + 25 µg, Flutiform (FP-FF) 125 + 5 µg (n = 9; mean ± st.dev;*p < 0.05;**p < 0.01;***p < 0.001).
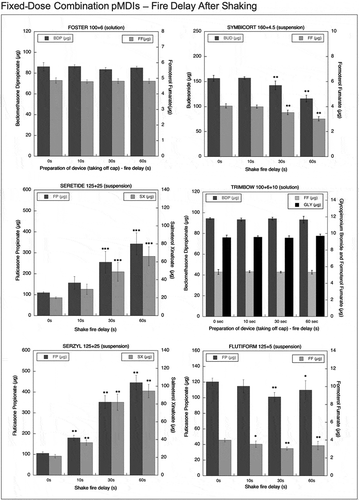
Figure 6. Emitted dose (µg) applying increasing time delays after shaking for high dose strength fixed-dose combination (HD_FDC) pMDIs: Foster (BDP-FF) 200 + 6 µg, Seretide (FP-SX) 250 + 25 µg, Serzyl (FP-SX) 250 + 25 µg, Flutiform (FP-FF) 250 + 10 µg (n = 9; mean ± st.dev;p < 0.05;**p < 0.01; ***p < 0.001).
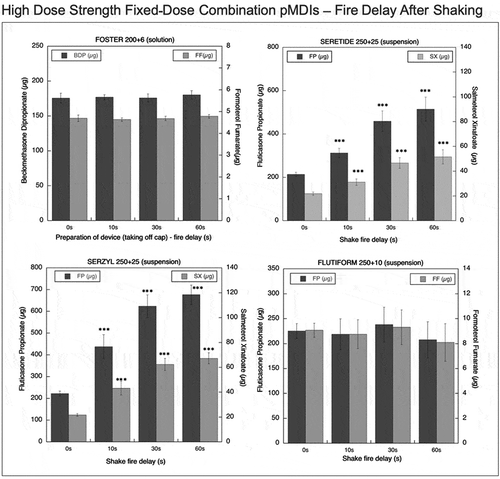
Figure 7. Emitted dose (µg) applying increasing time delays after shaking for monocomponent pMDIs: Clenil (100 µg BDP), Aircort (200 µg BUD), Ventolin (100 µg SS) and Flixotide (50 µg FP) (n = 9; mean ± st.dev; p < 0.05; **p < 0.01; ***p < 0.001).
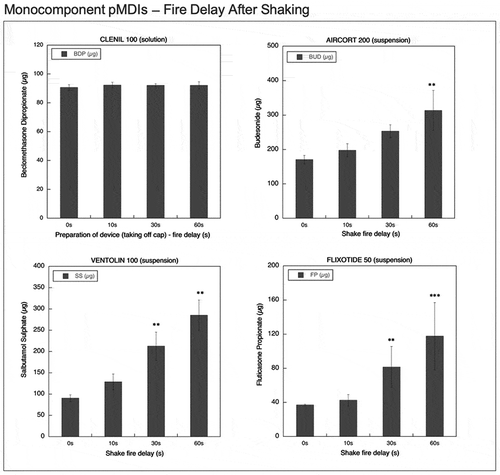
Figure 8. Overall summary data obtained in this study: emitted dose value of each API, released by a fixed-dose, high dose strenght fixed-dose combinations or monocomponent formulation, expressed as percentage in relation to the label claim. The data were collected during canister life cycle without shaking. The number of plotted values for each product were #135, #90 or #45 for triple-, double- or mono-component MDI product, respectively.
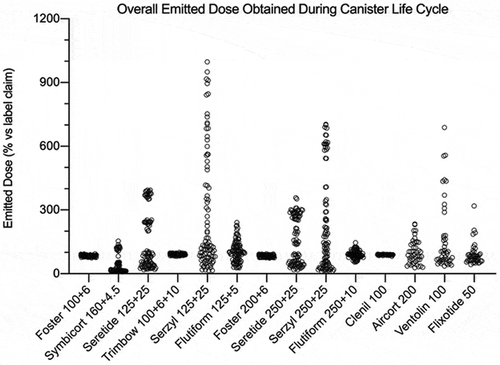
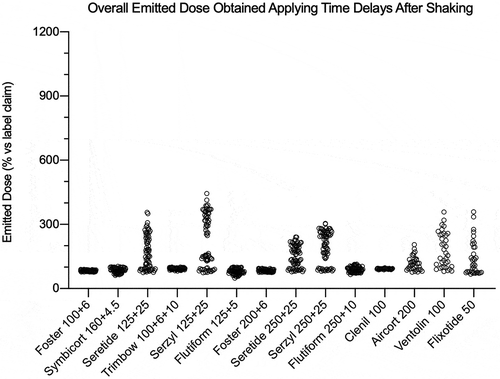
Figure 9. Overall summary data obtained in this study: emitted dose value of each API, released by a fixed-dose, high dose strenght fixed-dose combination or monocomponent formulation, expressed as percentage in relation to the label claim. The data were collected applying time delay after shaking. The number of plotted values for each product were #117, #72, or #36 for triple-, double- or mono-component MDI product, respectively.