Figures & data
Table 1. Use scenarios and knowledge tasks.
Table 2. Participant demographics.
Figure 2. Analysis of overall use errors (related to critical task) with potential for harm.
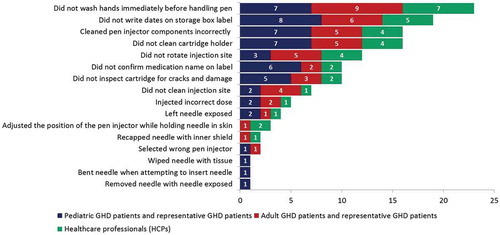
Figure 3. Total number of use errors on critical tasks per user group.
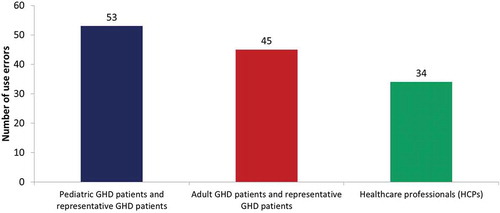
Figure 4. Number of observed errors with potential for harm in scenario 1 and scenario 3.
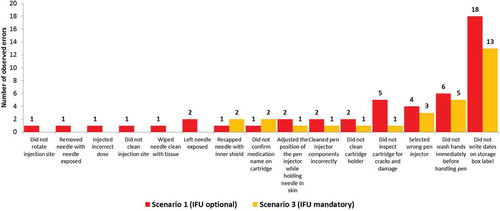
Table 3. Analysis of the use errors, root causes and action taken.

