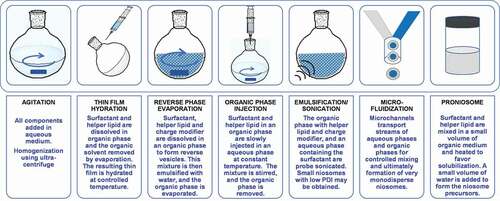
Figures & data
Table 1. Administration routes for delivery of drugs to the eye
Table 2. Some active substances of medicines used to reduce the IOP. Data from the European Medicines Agency, https://www.ema.europa.eu/en/medicines
Figure 1. Regional distribution of clinical trials related to diabetic eye. Data source: ClinicalTrials.gov. There were 657 outcomes for ‘diabetic eye’ on February 2021. Applied filters were Recruiting, Active not recruiting, Completed, Enrolling by invitation, and Terminated
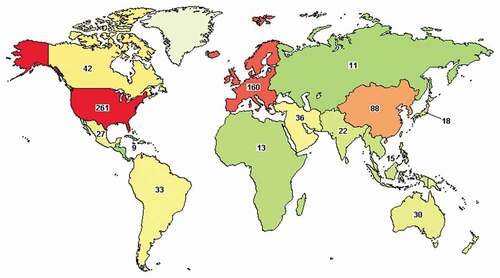
Table 3. Pharmacological treatments in clinical trials for diabetes-related macular edema classified as a function of the administration route, drug/biologic active substance, and number of clinical studies
Table 4. Pharmacological treatments in clinical trials for diabetes-related retinopathy classified as a function of the administration route, drug/biologic active substance, and number of clinical studies
Figure 2. Dependence of the architecture of the self-assembled nanocarrier on the critical packing parameter (CPP)
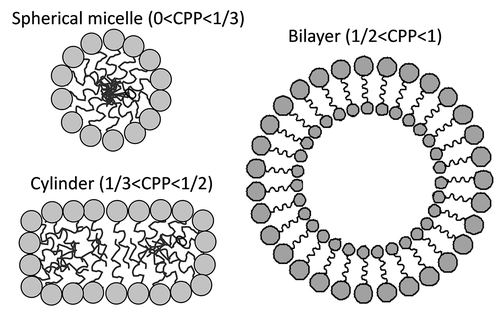
Figure 3. Progesterone (PG) apparent solubility in Soluplus and Pluronic F68 micelles, and permeability coefficients of cornea and sclera recorded for PG encapsulated in Soluplus 20% micelles or Pluronic F68 20% micelles. Reproduced from Alambiaga-Caravaca et al. [Citation126] (Creative Commons Attribution License)
![Figure 3. Progesterone (PG) apparent solubility in Soluplus and Pluronic F68 micelles, and permeability coefficients of cornea and sclera recorded for PG encapsulated in Soluplus 20% micelles or Pluronic F68 20% micelles. Reproduced from Alambiaga-Caravaca et al. [Citation126] (Creative Commons Attribution License)](/cms/asset/73d8f6e3-cc06-4169-81db-b9f6af591228/iedd_a_1953466_f0003_oc.jpg)
Table 5. Recent examples of self-assembled nanocarriers proposed for the topical ocular treatment of diabetic eye
Figure 4. (a) Central foveal thickness (CFT), (b) best-corrected visual acuity (BCVA), and (c) intraocular pressure (IOP) recorded for patients with refractory pseudophakic cystoid macular edema during treatment with triamcinolone-loaded liposomes (one drop every 2 h for 90 days). Reproduced from Gonzalez-De la Rosa et al. [Citation139] (Creative Commons License)
![Figure 4. (a) Central foveal thickness (CFT), (b) best-corrected visual acuity (BCVA), and (c) intraocular pressure (IOP) recorded for patients with refractory pseudophakic cystoid macular edema during treatment with triamcinolone-loaded liposomes (one drop every 2 h for 90 days). Reproduced from Gonzalez-De la Rosa et al. [Citation139] (Creative Commons License)](/cms/asset/ec8296d3-a106-4ec6-8505-756fe23b3517/iedd_a_1953466_f0004_b.gif)
Figure 6. Reduction in the intraocular pressure (IOP) observed after topical administration to normotensive rabbits of latanoprost-loaded niosome gel or conventional latanoprost eye drops. Error bars represent standard deviation of six replicates. Reproduced from Fathalla et al. [Citation167] with permission from Taylor & Francis (http://www.tandfonline.com)
![Figure 6. Reduction in the intraocular pressure (IOP) observed after topical administration to normotensive rabbits of latanoprost-loaded niosome gel or conventional latanoprost eye drops. Error bars represent standard deviation of six replicates. Reproduced from Fathalla et al. [Citation167] with permission from Taylor & Francis (http://www.tandfonline.com)](/cms/asset/5bbf12a9-4420-473a-8856-3e3afb29664f/iedd_a_1953466_f0006_oc.jpg)