Figures & data
Figure 1. BD Intevia™ 1 mL push on-skin and single-use Disposable Autoinjector before use (left) and after use (right)
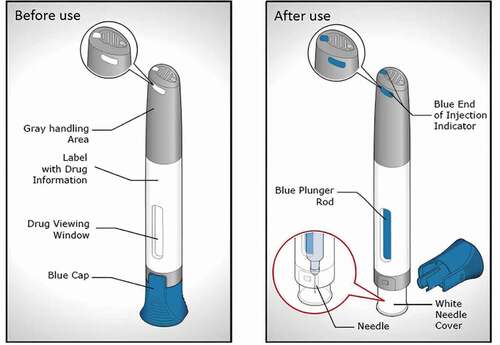
Figure 2. Visual, audible and tactile feedback indicators of BD Intevia™ 1 mL Disposable Autoinjector. The drug viewing window is empty before the activation of the device (a). Once the injection has started, indicated by a first click when pushing down the needle cover (b), the blue indicator progressively appears in the viewing window (c). The end of injection is signaled by three indicators, audible, visual and tactile at the top of the device (d). After the complete dose delivery, the drug viewing window appears fully blue (e)
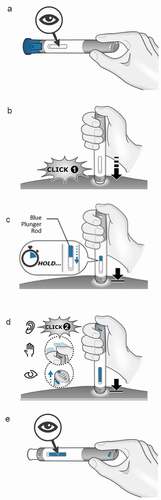
Figure 3. Summary of formative and validation study design. Abbreviations: HCP, Health Care Professionals; IFU, Instructions For Use
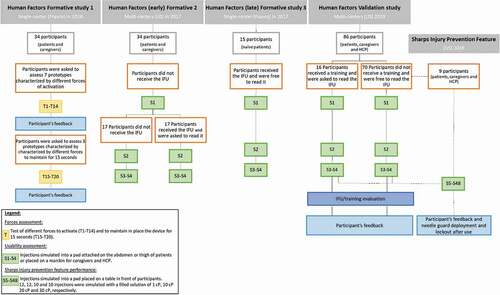
Table 1. List of essential and critical tasks performed by users during an injection. Critical tasks are highlighted in orange
Table 2. Demographic characteristics of participants. HCP: Health-Care Professionals
Figure 4. Activation forces acceptance when using BD Intevia™ 1 mL Disposable Autoinjector during the formative study 1. Reported acceptance toward different tested forces to activate the BD Intevia™ 1 mL Disposable Autoinjector during the formative study per participant (N total = 34). N, number of participants who rated activation force as ‘not acceptable,’ ‘neutral opinion’ or ‘acceptable.’ Note: The ‘very acceptable’ and ‘acceptable’ ratings are the two highest ratings on the Likert scale
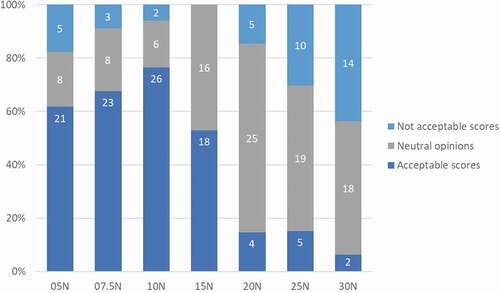
Figure 5. Usability of BD Intevia™ 1 mL Disposable Autoinjector during the early and late formative studies. a. Overall critical tasks completion rates per simulation for 34 participants of the formative study 2 performing six critical tasks and having read the IFU or not (N total of evaluated tasks per IFU group = 102). b. Overall critical tasks completion rates per simulation for 15 naïve self-injecting patients of the formative study 3 performing six critical tasks (N total of evaluated tasks = 90).
Rates of success, success with operational difficulties (SOD), close call and use error are represented. N, number of critical tasks performed.
*No participant read the IFU before the first simulation.
** NA for Not applicable was used for the tasks not performed because a blocking event on previous task
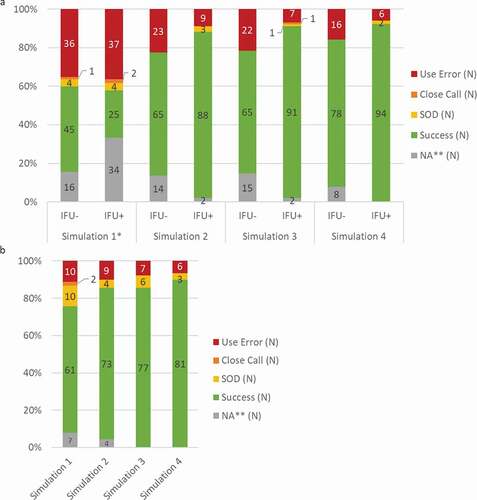
Table 3. Identified hazards and their mitigation during development of the BD Intevia™ 1 mL Disposable Autoinjector for subcutaneous administration
Figure 6. Usability of BD Intevia™ 1 mL Disposable Autoinjector during the validation study.
a. Overall injection success, success with difficulties (SOD), close call, and use error rates across the four simulated injections in the untrained and trained arms during the validation study. The six critical tasks were performed by 70 untrained and 16 trained participants. Two untrained participants performed an additional simulated injection due to product replacement (N total of evaluated tasks = 282).
b. Success, success with difficulties (SOD), close call, and use error rates per critical tasks across the four simulated injections during the validation study for 16 trained participants and 70 untrained participants performing six critical tasks. Two untrained participants performed an additional simulated injection due to product replacement (N total of evaluated tasks = 64 for the trained arm and N total of evaluated tasks = 282 for the untrained arm).
N, number of critical tasks performed.
* NA for Not applicable was used for the tasks not performed because a blocking event on previous task.
** Task 11: 172 tasks for simulated injections 3 and 4 (32 tasks from the trained group and 140 tasks from the untrained group) were not evaluated and rated NA. These 172 devices were included in the Sharps Injury Prevention Feature study
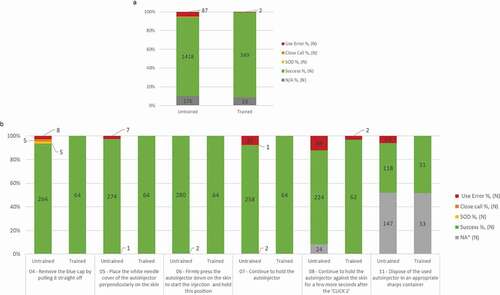
Figure 7. Reported understanding of the IFU and training during the validation study. N, number of participants who rated the IFU (N total = 86) or the training (N total = 16) as ‘very easy to understand’ and ‘easy to understand,’ ‘neutral opinion,’ or ‘difficult to understand’ and ‘very difficult to understand.’
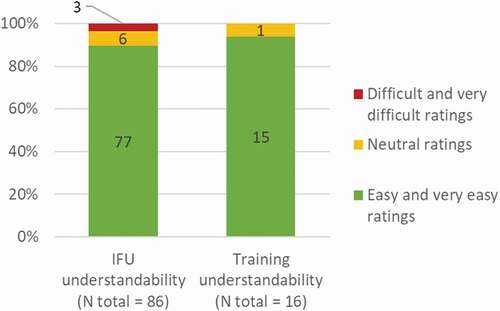
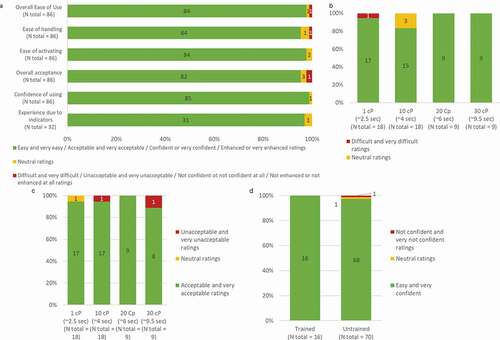
Figure 8. User-feedback scores on usability questions asked at the end of the validation simulated-use test. a. Ease of use, ease of handling, ease of activating, acceptance, confidence of using and experience due to the feedback indicators when using the BD Intevia™ 1 mL Disposable Autoinjector. Ease of use (b) and acceptance (c) toward different injection time when using the BD Intevia™ 1 mL Disposable Autoinjector. d. Reported confidence in controlling the start of injection during the validation study