Figures & data
Figure 1. Platelet-cancer interactions. The tumor microenvironment attracts platelets followed by binding and activation of the platelets by cancer cells. Platelets may be activated through multiple pathways including secretion of soluble mediators or direct receptor stimulation yielding functional advantages for the cancer cells.
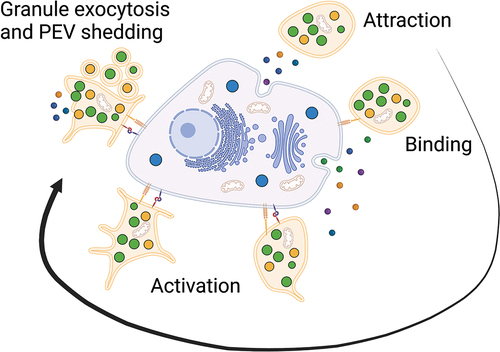
Figure 2. Platelet-assisted immune evasion. Platelet-cancer interactions assist cancer cells in evading NK and T cell cytotoxicity partially through TGF-ß-mediated mechanisms. In addition, binding of platelets also leads to pseudo-expression of MHC-1 and PD-L1, thus cancer cells may avoid recognition by NK cells through expression of platelet self-antigens and impair T cell cytotoxicity through checkpoint inhibition.
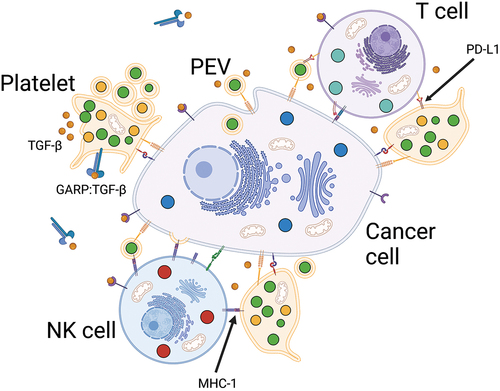
Figure 3. Platelets as drug-delivery systems. Platelets can be used as drug-delivery systems mainly through three different strategies. a) by drug-loading where platelets encapsulate the drug, then accumulate in the tumor microenvironment followed by activation and drug release. b) by drug-binding where large molecules such as antibodies or cytotoxic complexes are conjugated to the surface and kill cancer cells by cell-to-cell contact or by shedding of PEVs. c) by implementing platelet membranes in hybrid vesicles through fusing of PEVs with synthetic drug-loaded nanocarriers or other cell membranes to slow macrophage clearance and increase accumulation in the tumor microenvironment.
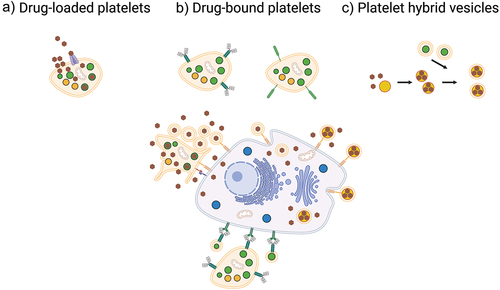
Figure 4. Strategies to optimize platelet-based drug-delivery systems. In order to enhance therapeutic efficacy, drug-loaded platelets, drug-bound platelets, or platelet hybrid vesicles can be conjugated with attachment molecules to increase retention in tumors a). Platelet infiltration of tumors may also be increased through combination with PTT b), tumor vascular-disrupting agents c), and tumor-inflammation increasing agents d). NIR, near infrared.
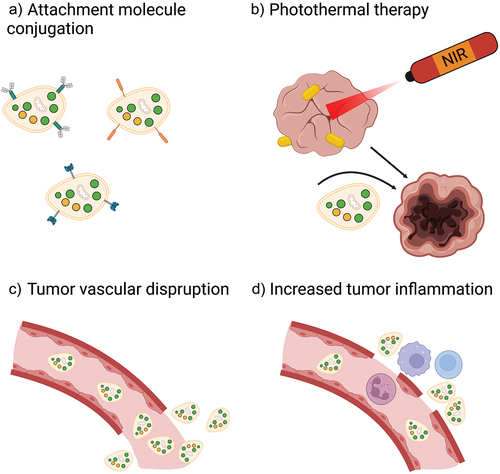
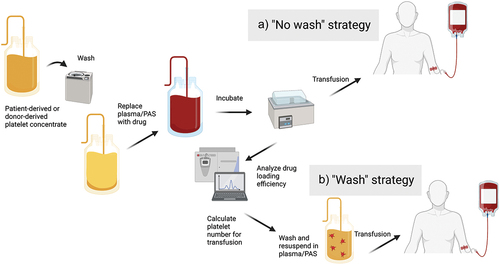
Figure 5. Clinical implementation of platelet-based drug-delivery systems. Platelets may be harvested from the patients themselves by thrombapheresis or supplied from blood bank storage. Following washing and resuspension in drug solution for loading or binding of drug, platelets are incubated and either transfused immediately a) or after washing and quantitation to calculate equivalent doses of free drug b). PAS, platelet additive solution.