Figures & data
Figure 1. Illustration of user steps with a prefilled handheld autoinjector activated by push-on-skin. a. The user pulls the needle cap straight off and holds the autoinjector against the skin. b. The user holds the autoinjector down until the injection is complete (i.e. until the second click is heard and the plunger rod filled the viewing window).
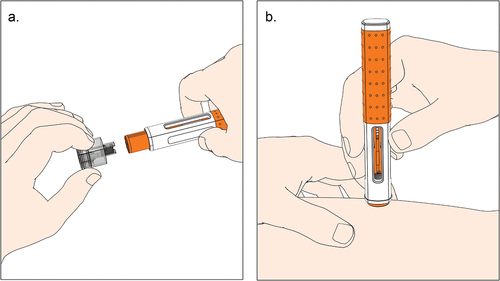
Table 1. Approved products from the U.S. Food and Drug Administration and European Medicines Agency using 2.25 mL prefilled syringes, prefilled needle safety devices, and prefilled handheld autoinjectors×.
Table 2. An overview of investigational large-volume handheld autoinjectors with pre-filled syringes and cartridges exceeding 2.25 mL capacity.
Figure 2. Evolution of previous research on large-volume subcutaneous drug delivery (N = 31), organized by three themes: injection tolerability, suitability for self-administration, and pharmacokinetic equivalence.
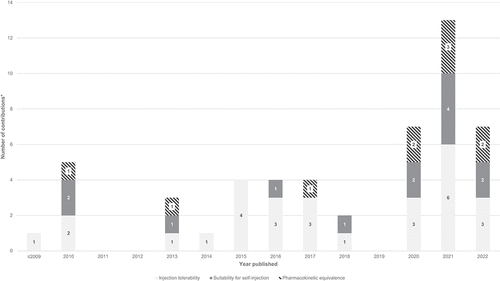
Figure 3. Overview of injection volume-rate ranges addressed by previous work and volume-rate ranges applicable to different drug delivery device categories.
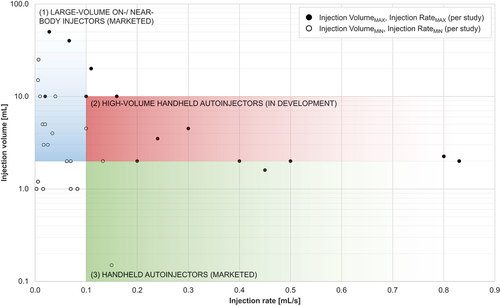
Table 3. Summary of literature review.
Table 4. A summary of review results for injection tolerance themes, including injection-related pain, injection-site reactions, and adverse events.
Table 5. A summary of review results for pharmacokinetic equivalence theme.
Table 6. Proposed future research topics classified by theme.
