Figures & data
Table 1. Baseline characteristics and first intake characteristics at index for all patients providing RebiSmart® usage data.
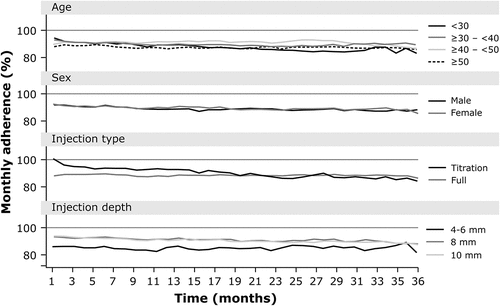
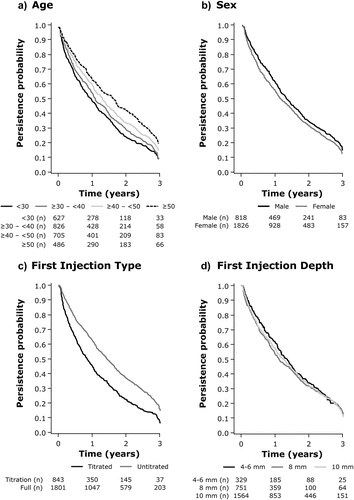
Table 2. Average adherence to, and duration of persistence with, the RebiSmart® device by category.
Table 3. Multivariate analyses of the association of sex, age, injection type, and injection depth with rates of adherence to, and duration of persistence with, the RebiSmart® device.
REB_RebiSmart_MDdialog_Manuscript_Suppl._Table_1.docx
Download MS Word (14 KB)Data availability statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.