Figures & data
Figure 1. Redox reactions and generation of reactive oxygen species following the reduction of apaziquone by one electron oxidoreductases. The quinone nucleus of apaziquone is reduced by NAD(P)H dependent one electron oxidoreductases such as cytochrome P450 reductase to a semiquinone radical which redox cycles back to the parent quinone in the presence of oxygen generating superoxide anions. Hydrogen peroxide is generated via superoxide dismutase (SOP) mediated reactions and this in the presence of trace metals can lead to the formation of hydroxyl radicals and subsequent damage to cellular macromolecules.
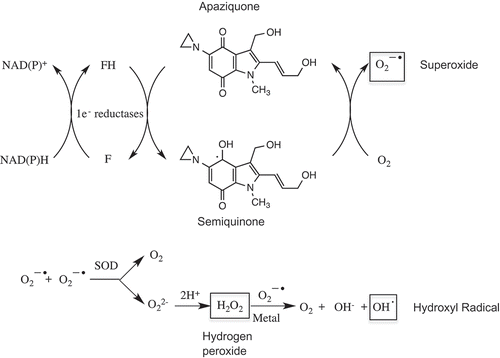
Figure 2. Mechanisms for DNA alkylation by apaziquone: (i) Pathways involving one- and two-electron reduction. Proton-coupled single-electron reduction of apaziquione generates stablised radical A, which can be further reduced (via B) to key intermediate C; C can be alkylated via cation D, formed by acid-catalysed dehydration, to give DNA monoadduct E. can undergo a second dehydration, giving dienyl cation F, which can be trapped in a second DNA alkyation event, giving interstrand cross-linked species G. (ii) Proton-accelerated mechanism. The conjugation of the aziridinyl lone-pair (acting as a vinylogous amide) into the quinone ring allows for an addition proton-accelerated DNA alkylation mechanism. Thus, protonation leads to cation A, which is in resonance with B; ring-opening of the reactive aziridinium moiety of B by DNA leads to aza-quinone species C, which can then undergo reduction to D. Acid-catalysed dehydration of D gives methylene indolium cation E, which can function as an active alkylating agent.
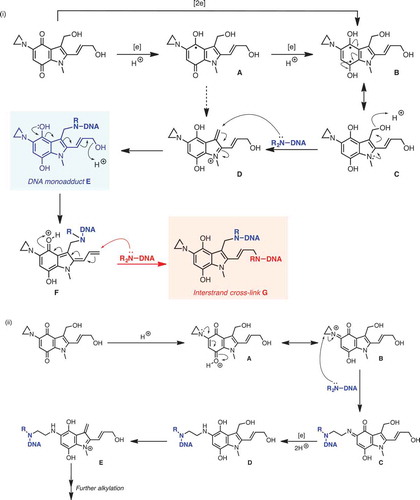
Table 1. In vivo antitumor activity of apaziquone (EO9) in combination with radiation in experimental rat tumors. The tumors were two squamous cell lung carcinomas (L17 and L42), a lung adenocarcinoma (L27), and a rhabdomyosarcoma (BA1112). All tumors were grown subcutaneously and EO9 was administered intraperitoneally (daily × 5 at 0.4 mg/kg). When EO9 was combined with 5 daily doses of 4Gy, additive or synergistic effects were observed. This data were originally reported by Kal et al [Citation53].
Figure 3. The presence of a marker lesion prior to (A) and after (B) a 6 week course of apaziquone. The results were first presented in the review article by Phillips et al, reproduced with permission [Citation6].
![Figure 3. The presence of a marker lesion prior to (A) and after (B) a 6 week course of apaziquone. The results were first presented in the review article by Phillips et al, reproduced with permission [Citation6].](/cms/asset/7c698142-d47a-4489-9303-378a16d693d8/iemt_a_1341490_f0003_oc.jpg)
Box 1. Drug summary box: apaziquone
