Figures & data
Figure 1. Illustration of the effects that metabolites have on the overall effect and toxicity of therapeutic agents. (a) Case of a hypothetical drug (D) that is metabolized into active, toxic, and inactive metabolites (Ma, Mt, and Mi, respectively). Corresponding time-profiles for concentrations, effects, and toxicities (arbitrary units) are shown for all components assuming that D is metabolized with different rates into these metabolites and they all have different elimination rates as well as activities and toxicities. For the case shown here, most of the effect is contributed by the original drug D and its active metabolite Ma, but most of the toxicity results from the toxic metabolite Mt. (b) Same for a hypothetical soft drug (SD) that is metabolized rapidly into a single inactive metabolite Mi of negligible activity and toxicity. Contrary to A, both activity and toxicity are driven only by those of the original SD even if Mi concentrations are quite high. Hence, complex PK profiles and toxicities due to reactive metabolites are avoided, and activity and toxicity can be better controlled. Time-profiles were obtained using single-compartment models with absorption, metabolism, and elimination rate constants (kabs, km,a, kel, etc.) and corresponding differential equations as indicated. All effects and toxicities were assumed to be directly proportional with concentrations (i.e. no effect compartment).
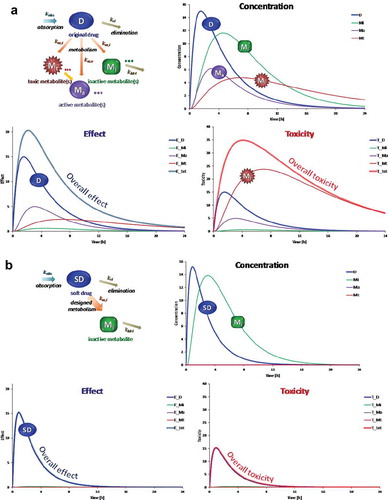
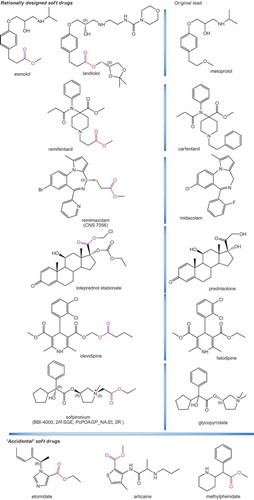
Table 1. Rationally designed or accidental soft drugs that are approved for clinical use (trademark provided) or have reached clinical development. For each compound, the entity responsible for its development, the year of the first publication, and the approved trademark (if available) are also included.