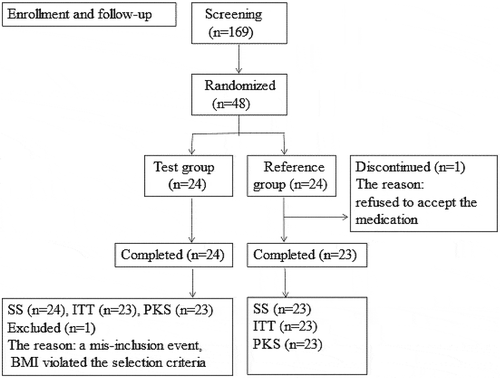
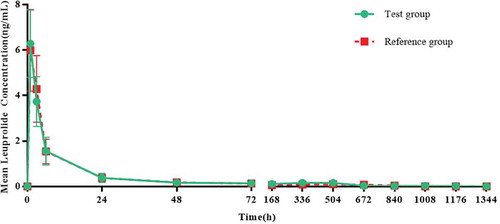
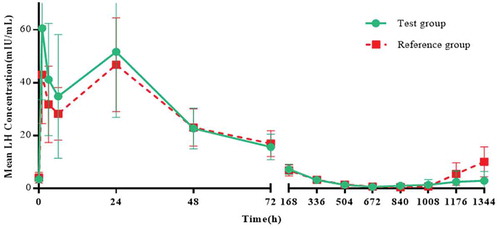
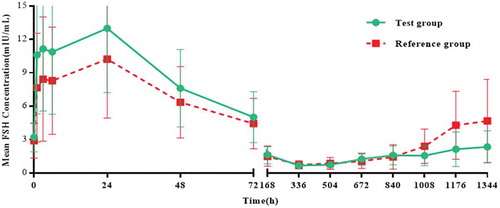
Figures & data
Table 1. Baseline characteristics (data set: ITT)
Table 2. The inferential analysis, the geometric mean ratio and the 90% CI of leuprolide pharmacokinetic parameters of the test group and the reference group (data set: PKS)
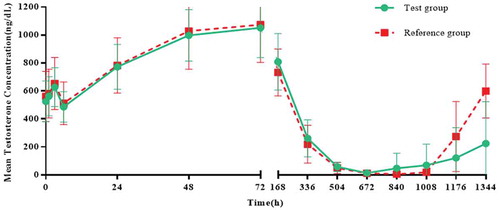
Table 3. Testosterone parameters (data set: ITT)
Table 4. Summary of adverse events (data set: SS)
Table 5. Summary of the relationship between TEAE and study drugs (data set: SS)