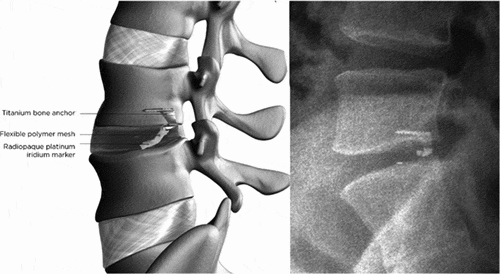
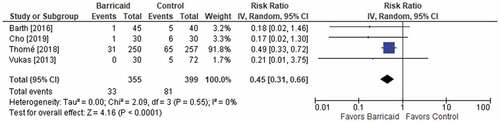
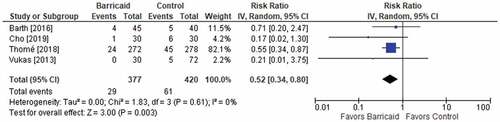
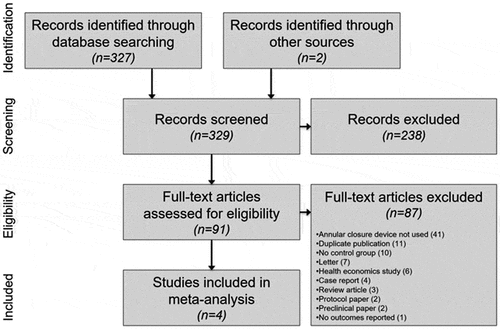
Figures & data
Table 1. Compendium of 50 peer-reviewed manuscripts of the Barricaid device for annulus fibrosus closure.
Table 2. Patient and study characteristics in controlled studies with the Barricaid device for annulus fibrosus closure.
Table 3. Risk of bias in controlled studies with the Barricaid device for annulus fibrosus closure.