Figures & data
Figure 1. Submillimeter targeting with tightly spaced electrodes and multiple current sources
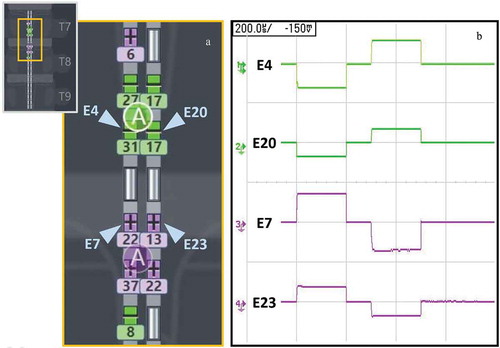
Table 1. Baseline demographic characteristics in analyzed patients (n/N)
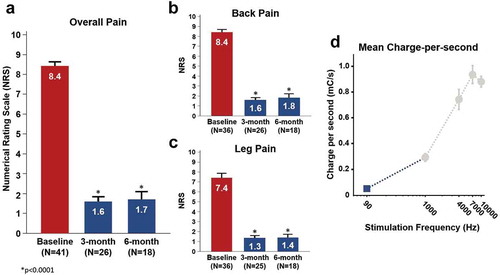
Figure 3. Pain reduction at initial programming session using FAST
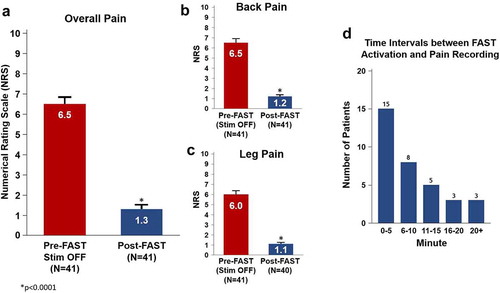
Figure 4. Pain reduction and charge expenditure using FAST