Figures & data
Figure 1. Carpal tunnel release with ultraGuideCTR and real-time ultrasound guidance. A. ultraGuideCTR device, consisting of hand piece and distal working tip. B. close-up view of device tip from ‘bird’s eye view’ demonstrating inflated balloons which increase the device diameter from 4 mm to 8 mm to create space within the carpal tunnel. C. cross-sectional view of the distal carpal tunnel with the device placed directly below the transverse carpal ligament (TCL) and between the median nerve (MN) and the hook of the hamate bone (HAM). the balloons (yellow asterisks) have been inflated to create space between the MN and the ulnar artery (UA). top = superficial, left = radial.
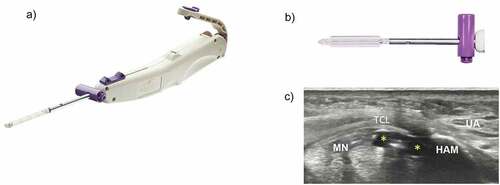
Table 1. Characteristics of patients treated using carpal tunnel release with ultrasound guidance
Figure 2. Change in QDASH score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 15 points [Citation14]. QDASH = Quick Disabilities of the Arm, Shoulder, and Hand Questionnaire.
![Figure 2. Change in QDASH score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 15 points [Citation14]. QDASH = Quick Disabilities of the Arm, Shoulder, and Hand Questionnaire.](/cms/asset/7cba9150-d2a1-4747-ae22-c02a9c398b97/ierd_a_2048816_f0002_b.gif)
Figure 3. Change in BCTQ-SSS and BCTQ-FSS score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are the absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 0.74 for BCTQ-FSS and 1.14 points for BCTQ-SSS [Citation15]. BCTQ-FSS = Boston carpal tunnel questionnaire functional status scale; BCTQ-SSS = Boston Carpal Tunnel Questionnaire Functional Status Scale, and Boston Carpal Tunnel Questionnaire Symptom Severity Scale.
![Figure 3. Change in BCTQ-SSS and BCTQ-FSS score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are the absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 0.74 for BCTQ-FSS and 1.14 points for BCTQ-SSS [Citation15]. BCTQ-FSS = Boston carpal tunnel questionnaire functional status scale; BCTQ-SSS = Boston Carpal Tunnel Questionnaire Functional Status Scale, and Boston Carpal Tunnel Questionnaire Symptom Severity Scale.](/cms/asset/02a93d17-056a-4dfb-8bf4-fc72e70eacf0/ierd_a_2048816_f0003_b.gif)
Table 2. Change in main outcomes reported as a standardized effect size over 6 months following carpal tunnel release with ultrasound guidance.a.
Figure 4. Percentage of patients who returned to normal activities following carpal tunnel release with ultrasound guidance. median time to return to normal activities was 3 days (interquartile range: 2–5 days).
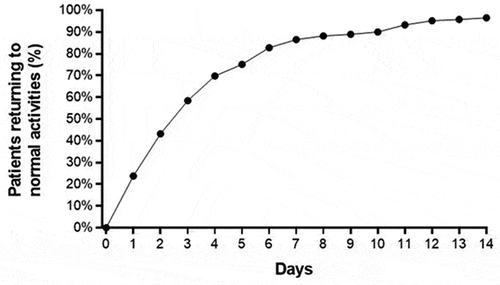
Figure 5. Percentage of employed patients who returned to work following carpal tunnel release with ultrasound guidance. median time to return to work was 5 days (interquartile range: 3–9 days).
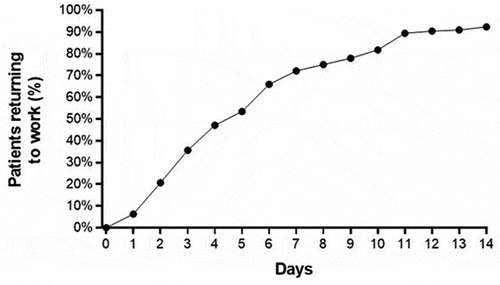
Table 3. Subgroup analysis of main outcomes following carpal tunnel release with ultrasound guidance
Table 4. Comparison of results using carpal tunnel release with ultrasound guidance vs. open/mini-open carpal tunnel release
Supplemental Material
Download MS Word (12.1 KB)Data Availability Statement
Raw data will not be made available since this is an ongoing registry.
