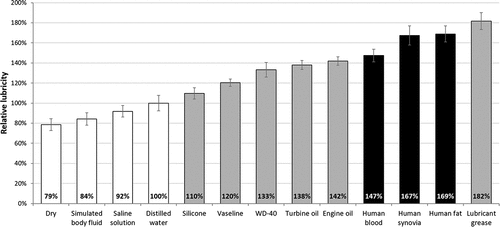
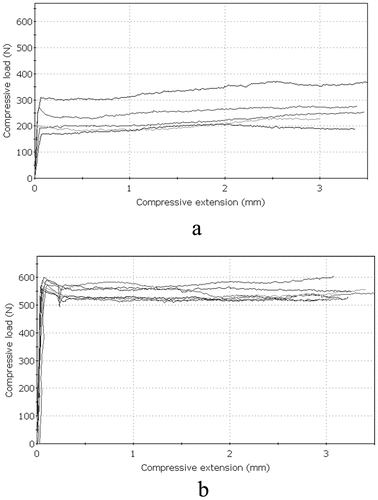
Figures & data
Figure 1. The schematic drawing of the device developed for lubricity measurements. 1. compression force by spring. 2. moving force. 3. contact surface.
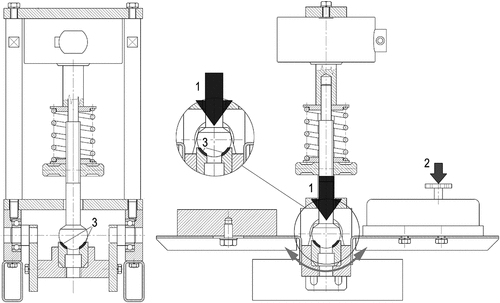
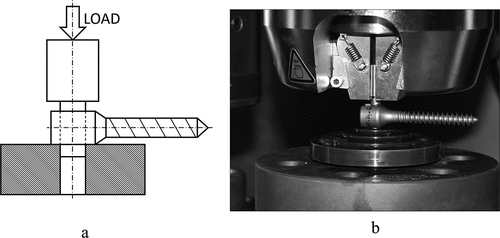
Figure 2. The scheme of the setup of stability measurements (a) and using an Instron 8874 material testing machine (b) according to the ASTM 1798–97 (2008) standard setup.