Figures & data
Table 1. Baseline characteristics (n = 189). AF: atrial fibrillation, BMI: body mass index, CTI: cavotricuspid isthmus ablation, Extended AF-ablation: Including substrate modification, lines and/or complex fractionated atrial electrograms (CFAE), PVI: Pulmonary vein isolation, SD: standard deviation.
Figure 1. The device is connected by Bluetooth to a smartphone. 30-second chest and 30-second thumb registrations are collected. Permission to use approved by Coala Life AB.
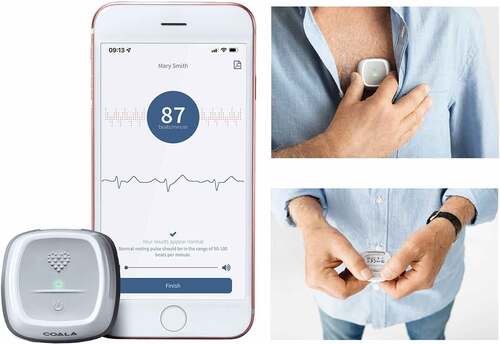
Figure 2. Chest-and thumb registrations during sinus rhythm and atrial fibrillation. Permission to use approved by Coala Life AB.
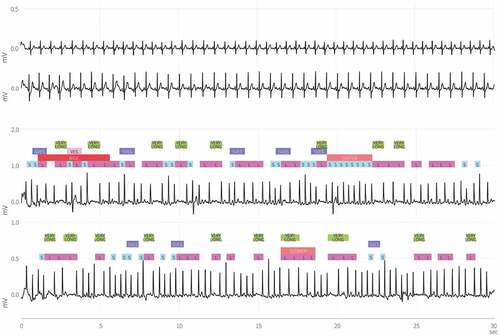
Figure 3. Study flow diagram. AF: atrial fibrillation, AFL/AT: atrial flutter/atrial tachycardia, ATA: atrial tachyarrhythmia, CHM: Coala Heart Monitor.
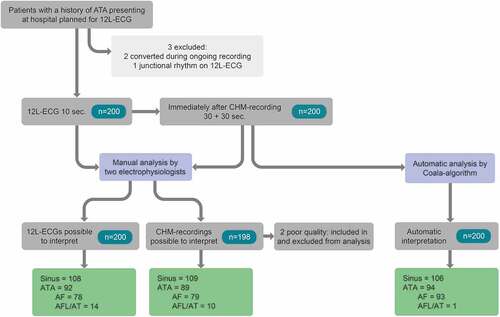
Table 2. Accuracy values of CHM~TC~
