Figures & data
Table 1. Definitions.
Table 2. Definitions of artificial intelligence in reports and regulatory documents with relevance for medical devices.
Figure 1. Comparison of regulatory definitions of medical device software and their scope.
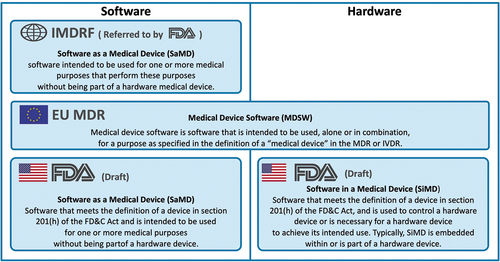
Table 3. Factors influencing the risks of software as a medical device (SaMD).
Table 4. Cross-specialty recommendations on medical AI, developed using a systematic expert consensus methodology.
Table 5. Cross-specialty or specialty-specific recommendations on medical AI, published by medical associations or groups of investigators.
Table 6. Key components of consensus-based guidelines.
Figure 2. A simplified landscape of the inter-relationships of European and global bodies engaged in the development of standards for AI medical devices.
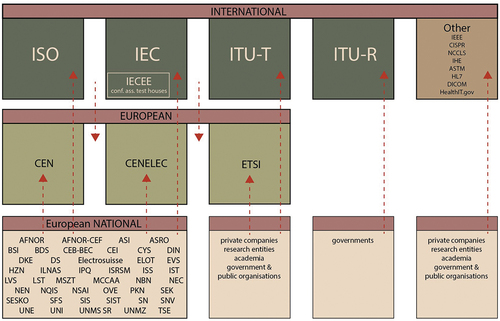
Table 7. Information technology standards relating to general aspects of AI, that have already been published.
Table 8. General standards under joint development by the International Standardization Organization (ISO) and the International Electrotechnical Commission (IEC), related to artificial intelligence.
Table 9. Selected regulatory initiatives and proposals under development.
Figure 3. Overview of European and global institutions and organizations engaged in the development of regulations for medical AI systems.

