Figures & data
Figure 1. Compilation of two Adverse Outcome Pathways (AOPs) leading to effects on the liver by TiO2. Events resulting in the adverse outcomes liver fibrosis, steatosis and edema are based on the AOP 144 on liver inflammation of the AOP-Wiki (https://aopwiki.org/aops/144). Adaptations based on recent studies, AOP 34 with hepatic steatosis as adverse outcome (https://aopwiki.org/aops/34) and expert judgment, are included. After the molecular initiating event (MIE), a series of key events (KE) take place that lead to an adverse outcome (AO) or and associated event (AE). ECM: extra-cellular matrix; HSC: hepatic stellate cell; ROS: reactive oxygen species.
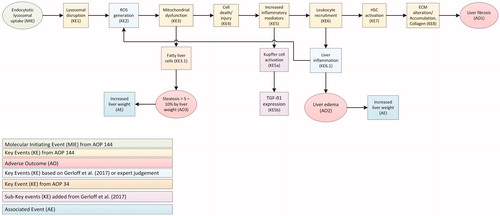
Table 1. Overview of the oral in vivo studies with TiO2 investigating the suggested adverse outcome pathway (AOP) leading to liver fibrosis, steatosis and edema, expressed against dose level ().
Figure 2. Adverse Outcome Pathways (AOP) for the intestine leading to tumor formation as a result of oral TiO2 exposure. The AOP has been postulated by Braakhuis et al. (Citation2021) for TiO2 related colon carcinogenicity after oral exposure. After the molecular initiating event (MIE), a series of key events (KE) take place that lead to the adverse outcome (AO). ROS: reactive oxygen species.
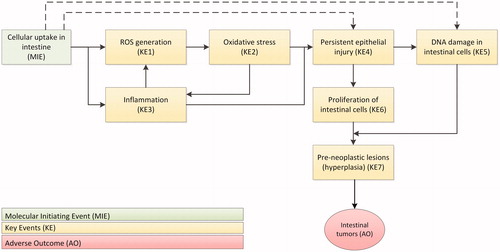
Table 2. Overview of oral in vivo studies investigating the suggested adverse outcome pathway (AOP) leading to TiO2 related colon carcinogenicity after oral exposure in rats.
Table 3. Overview of oral in vivo studies investigating the suggested adverse outcome pathway (AOP) leading to TiO2 related colon carcinogenicity after oral exposure in mice.
Table 4. Gender and age of the subjects from Heringa et al. (Citation2018) and Peters et al. (Citation2020), and of the combined studies.
Table 5. Total Ti levels in postmortem human organs/tissues from Heringa et al. (Citation2018) and Peters et al. (Citation2020), as well as combined total Ti levels (for liver and spleen).
Table 6. Significant different (from the control value mentioned) Ti levels in liver, spleen, kidney and intestinal tissues as a result of different doses of TiO2 as reported in different animal studies with oral exposure (duration of the study between parentheses; d: day, w: week).
