Figures & data
Figure 1. Branches of science underpinning ULES. The combination of four components: liquid, powder, heat and pressure, requires a multi-disciplinary approach covering physics, chemistry, geoscience and biology. A synergistic knowledge-base, built up from a wide range of disciplines, ranging from material science and engineering to palaeontology [Citation5], could aid the understanding of these techniques to support their technological and scientific impact.
![Figure 1. Branches of science underpinning ULES. The combination of four components: liquid, powder, heat and pressure, requires a multi-disciplinary approach covering physics, chemistry, geoscience and biology. A synergistic knowledge-base, built up from a wide range of disciplines, ranging from material science and engineering to palaeontology [Citation5], could aid the understanding of these techniques to support their technological and scientific impact.](/cms/asset/cc0bb5b9-91e7-4438-975a-f45ce590fa03/yaac_a_1706825_f0001_oc.jpg)
Figure 2. (a) Wollaston Press developed in 1829 for compaction of loose platinum mud into a firm pellet [Citation7]. (b) Extract from early work by Brill and Melczynski dated 1964 [Citation8] on hydrothermal sintering. At the time, the authors did not intend to continue studies because they could not find any useful application. (c) Setup developed by Turba and Rump [Citation9] for carrying out ‘cold sintering’ experiments: a systematic correlation between strength of barite pellets and amount of water was found.
![Figure 2. (a) Wollaston Press developed in 1829 for compaction of loose platinum mud into a firm pellet [Citation7]. (b) Extract from early work by Brill and Melczynski dated 1964 [Citation8] on hydrothermal sintering. At the time, the authors did not intend to continue studies because they could not find any useful application. (c) Setup developed by Turba and Rump [Citation9] for carrying out ‘cold sintering’ experiments: a systematic correlation between strength of barite pellets and amount of water was found.](/cms/asset/91efe553-3780-4934-9b4f-380d5fa2928e/yaac_a_1706825_f0002_oc.jpg)
Figure 3. Road map tracing milestones in near room temperature consolidation (T < 300 °C) over the past four decades. Several techniques have been developed, based on dry (top) and wet (bottom) ULES [Citation11–74].
![Figure 3. Road map tracing milestones in near room temperature consolidation (T < 300 °C) over the past four decades. Several techniques have been developed, based on dry (top) and wet (bottom) ULES [Citation11–74].](/cms/asset/61eb3bcf-dcf4-48a1-9398-0952fa4c0c1f/yaac_a_1706825_f0003_oc.jpg)
Figure 4. Experimental setups used for ULES. The main differences are presented in . (a) CS, (b) CSP, (c) HHP, and (d), rHLPS.
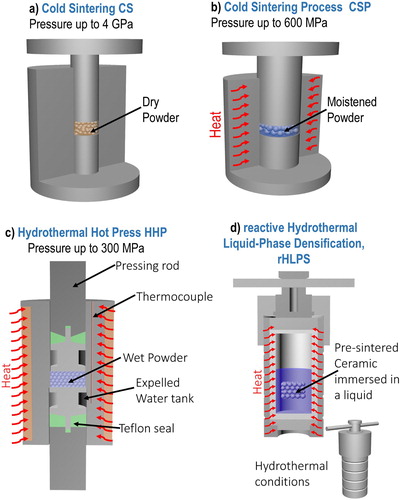
Figure 5. Number of publications on ULES per year, as of June 2019: (a) in dry conditions using CS; (b) in the presence of a liquid using HHP and CSP.
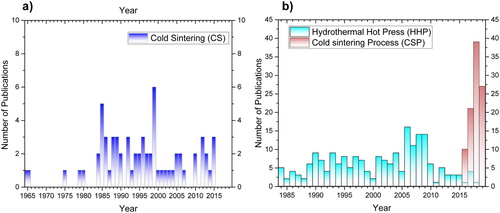
Table 1. Comparative analysis of ULES techniques.
Table 2. Primary bond characteristics in dry and wet conditions. A similar table, proposed by Alberts et al. in a biology textbook [Citation167], is useful to understand the formation of primary bonds in ULES.
Table 3. Processing route, solubility, ionic species.
Figure 6. (a) Aqueous geochemistry and speciation of ‘Hard’ (also referred to as ‘Type A’) Cations (All electrons removed from outer shell). Extract of periodic table from an Earth Scientist’s Periodic Table [Citation229]. Ionic potentials follow the order F > O>N = Cl > Br > I>S (isolines). (b) Logarithm of the solubility of their oxide in water at 25 °C [Citation229].
![Figure 6. (a) Aqueous geochemistry and speciation of ‘Hard’ (also referred to as ‘Type A’) Cations (All electrons removed from outer shell). Extract of periodic table from an Earth Scientist’s Periodic Table [Citation229]. Ionic potentials follow the order F > O>N = Cl > Br > I>S (isolines). (b) Logarithm of the solubility of their oxide in water at 25 °C [Citation229].](/cms/asset/2948fe86-8212-4436-9ee4-8834de65c1ff/yaac_a_1706825_f0006_oc.jpg)
Figure 7. Cumulative number of papers of typical materials processed using CS, HHP and CSP. According to Marie et al. [Citation136], more than 50 materials have been processed using CSP. Considering the chemical composition presented in and their combinations (metals/ceramics and organics/ceramics) the list of materials processable by ULES could become endless.
![Figure 7. Cumulative number of papers of typical materials processed using CS, HHP and CSP. According to Marie et al. [Citation136], more than 50 materials have been processed using CSP. Considering the chemical composition presented in Table 1 and their combinations (metals/ceramics and organics/ceramics) the list of materials processable by ULES could become endless.](/cms/asset/23f1e65d-27ca-4f3e-8ab4-0760e31ab468/yaac_a_1706825_f0007_oc.jpg)
Figure 8. An overview of the room temperature consolidation mechanisms in metals and ceramics: (a) Hertizian contact between two sintering particles showing an increased stress at the contact point which might contribute to increased solubility at the neck during ULES; (b) Application of the Panneli and Filho equation applied to several dry pressed materials (adapted from [Citation179]); (c) formation of a hydrated layer during HHP of silica (taken from [Citation244] and re-printed with the permission of the editor); (d) sintering kinetics during CSP of ZnO showing plastic flow sintering (adapted from [Citation245]); (e) and (f) grain growth kinetics in ZnO during CSP, indicating that grain boundary diffusion is activated even at low temperature (adapted from [Citation204]).
![Figure 8. An overview of the room temperature consolidation mechanisms in metals and ceramics: (a) Hertizian contact between two sintering particles showing an increased stress at the contact point which might contribute to increased solubility at the neck during ULES; (b) Application of the Panneli and Filho equation applied to several dry pressed materials (adapted from [Citation179]); (c) formation of a hydrated layer during HHP of silica (taken from [Citation244] and re-printed with the permission of the editor); (d) sintering kinetics during CSP of ZnO showing plastic flow sintering (adapted from [Citation245]); (e) and (f) grain growth kinetics in ZnO during CSP, indicating that grain boundary diffusion is activated even at low temperature (adapted from [Citation204]).](/cms/asset/d48ffb07-8ca8-4663-8871-8214b017232b/yaac_a_1706825_f0008_oc.jpg)
