Figures & data
Figure 1. Basic CRISPR/Cas9 systems for allergic and immunologic diseases. (a) Conventional CRISPR/Cas9 system for gene editing. sgRNA and Cas9 form a ribonucleoprotien complex. sgRNA target sequence is complementary to a specific genomic location and allows binding of the RNP at that loci. Cas9 then creates a double-strand break. Cellular DNA repair mechanisms repair the break. A proportion of these repairs will result in gene knockouts or, if a donor DNA sequence is provided, point mutations or large insertions. Donor DNA sequences contain the desired change flanked by regions homologous to the DNA sequence proximal and distal to the genomic mutation site. Deactivated Cas9 (dCas9) systems can be used to modulate gene expression. Various enhancers or repressors can be fused to Cas9 itself (b), or aptamer technology can be used to allow binding of an enhancer or repressor to the sgRNA (c). After the RNP binds to a specific locus, the enhancer or repressor can modulate the expression of a nearby gene. (d) Using CRISPR/Cas9-aptamer-based gene regulation, it should be possible to achieve multiplex modulation of the expression of transcription factors and cytokine mediators, allowing for repression of the Type 2 T helper (Th2) phenotype associated with atopic disease and promoting development of either a Type 1 T helper (Th1) or a regulatory T cell (Treg) response. Diagrammed are potential targets for such a system. For example, GATA binding protein 3 (GATA-3) is a transcription factor important in the development of Th2 cells and forkhead box p3 (FoxP3) is a transcription factor important in the development of Treg cells. Using CRISPR/Cas9 to repress (blue minus sign) GATA-3 or induce (orange plus sign) FoxP3 expression, it may be possible to skew T cell development away from Th2 development and towards Treg development, respectively. T-box transcription factor TBX21 (T-bet), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 13 (IL-13), interferon γ (IFN-γ), interleukin 2 (IL-2), cytotoxic T lymphocyte–associated protein 4 (CTLA-4), interleukin 10 (IL-10), transforming growth factor β (TGF-β), peripheral blood mononuclear cell (PBMC).
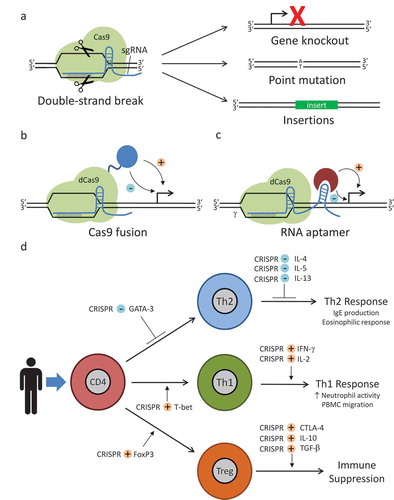
Table 1. Basic CRISPR/Cas9 systems with potential uses in clinical allergy and immunology.
