Figures & data
Table 1. Studies on DMF included
Table 2. Mean percentage improvements in regional PASI at week 16
Figure 1. Erythema – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 16 per body region
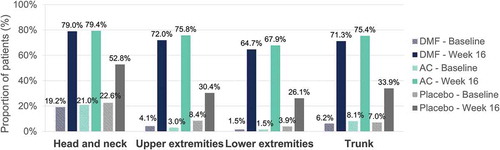
Figure 2. Induration – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 16 per body region
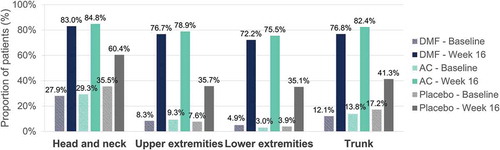
Figure 3. Desquamation – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 16 per body region
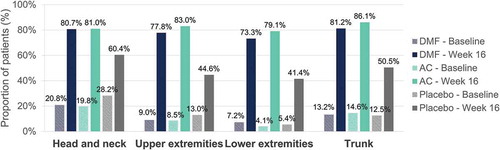
Table 3. Baseline characteristics
Table 4. Mean percentage improvements in regional PASI at week 24
Figure 4. Erythema – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 24 per body region
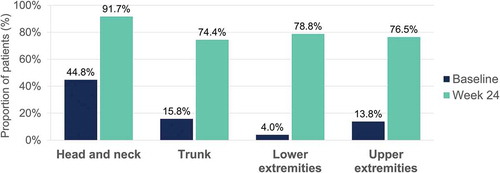
Figure 5. Induration – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 24 per body region
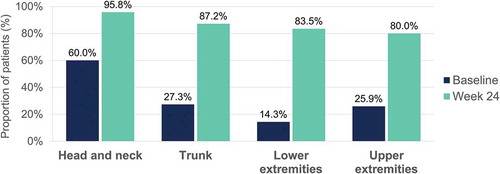
Figure 6. Desquamation – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 24 per body region
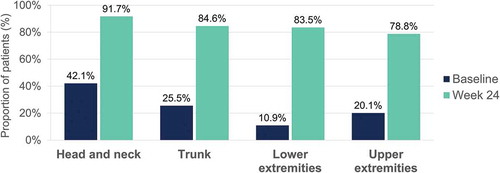
Table 5. Baseline characteristics
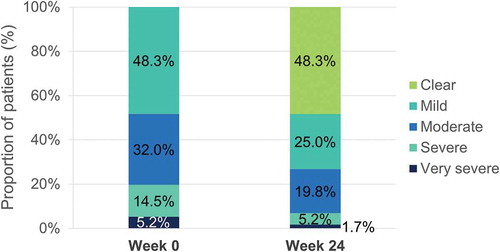
Figure 7. Evolution of median absolute PASI after 24 weeks of dimethyl fumarate treatment
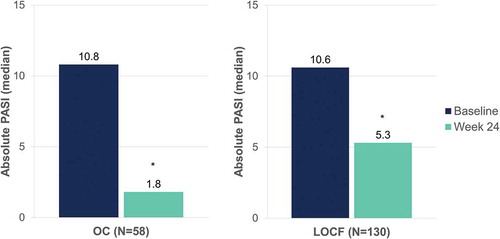
Figure 8. Evolution of median body surface area affected after 24 weeks of dimethyl fumarate treatment
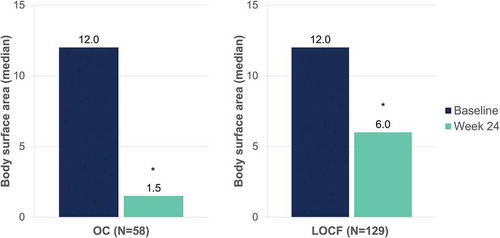
Figure 9. Evolution of median pruritus VAS scores after 24 weeks of dimethyl fumarate treatment
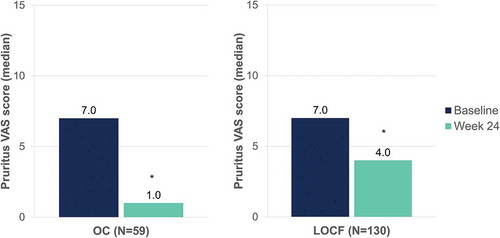
Figure 10. Evolution of median DLQI scores after 24 weeks of dimethyl fumarate treatment
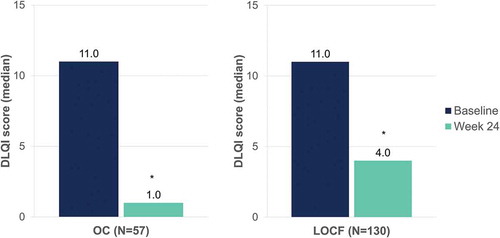
Table 6. Baseline characteristics
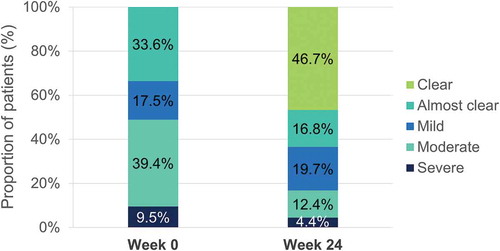
Figure 11. Evolution of absolute PASI <3 and <5 after 24 weeks of dimethyl fumarate treatment
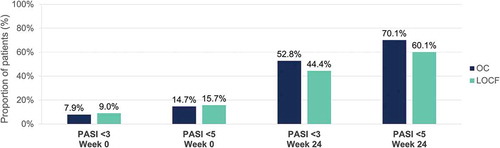
Figure 12. Evolution of scalp PGA 0 or 1 (clear or almost clear) after 24 weeks of dimethyl fumarate treatment
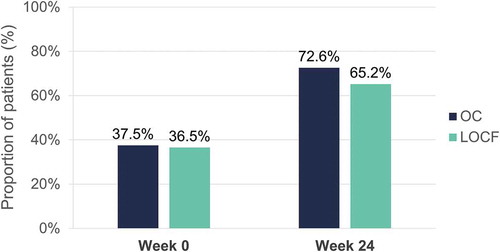
Figure 15. Evolution of itch (NRS <3) after 24 weeks of dimethyl fumarate treatment
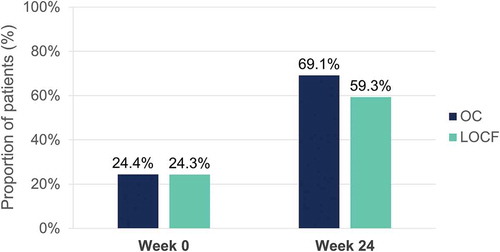
Table 7. Most frequent adverse events (>5 events)