Figures & data
Figure 1. CVID enteropathy. A suggested etiology model of CVID enteropathy where the size of the circles reflects the suggested impact on the complex enteropathy phenotype.
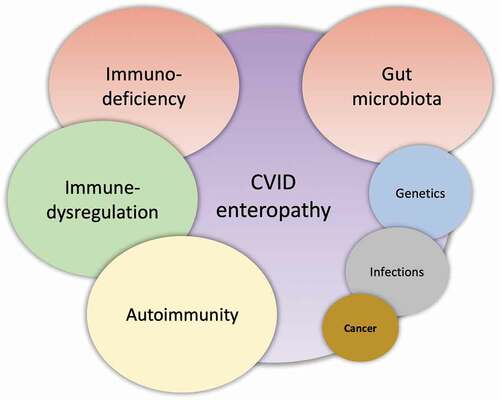
Table 1. An overview of some of the definitions used for CVID enteropathy
Figure 2. CVID enteropathy sub-classes. CVID enteropathy divided into the two subclasses: Severe CVID enteropathy and Non-severe CVID enteropathy.
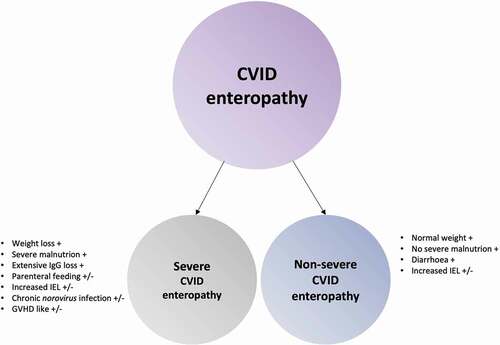
Figure 3. Macroscopic and microscopic gastrointestinal pathology and gut microbial dysbiosis in CVID. Gastrointestinal disease in CVID is associated with immune dysregulation, immunodeficiency and autoimmunity that effects the stomach, liver and large- and small intestine. The gut microbial composition in CVID is unhealthy and together with GI inflammation it allows gut leakage of microbial products into the blood stream to initiate/maintain an inflammatory process.
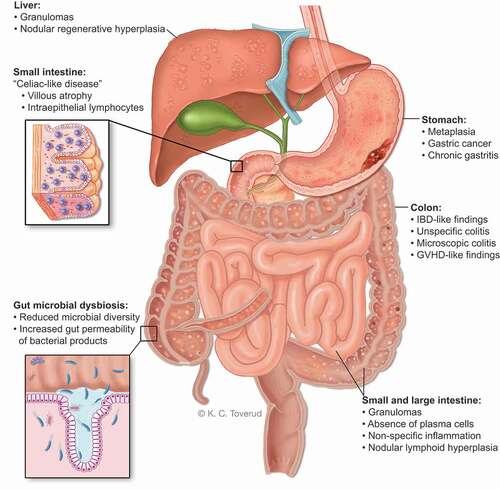
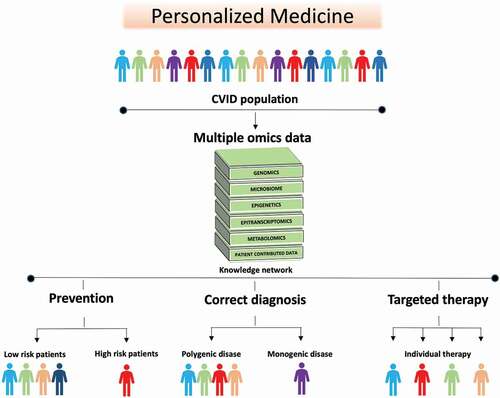
Figure 4. The future of CVID is personalized medicine. The heterogenous nature of CVID and the overlap between CVID enteropathy and inflammation/autoimmunity in other organs makes it logical to approach personalized therapy in CVID as a group. Data such as genomics, transcriptomics, metabolomics, and metagenomics including samples from blood, tissues and feces, are combined. Then statistical and mathematical integration methods for multiple omics datasets are applied to unravel the complexity of biological systems involved in CVID and to identify signatures that can stratify the patients further. This new knowledge can be used in: (i) Prevention, to detect patients at high risk of developing complications e.g. gastric cancer, liver failure or severe chronic enteropathy (ii) Correct diagnosis: to identify monogenic disease that enables individualized treatment strategy, and individuals that are wrongly categorized as CVID (iii) Targeted therapy: to predict which patients are more likely to respond to specific therapies e.g. biological therapy directed at specific mechanistic pathways, nutritional intervention e.g. high fiber diet, microbiome treatment e.g. probiotics, or a combination thereof.