Figures & data
Figure 1. The mitogen-activated signaling pathway, from left to right: a) normal function of MAPK pathway signaling; b) BRAFV600E mutation leading to constituently activated MAPK signaling and enhanced pathogenesis of melanoma; c) targeting of MAPK pathway, with BRAFi and MEKi, reducing melanoma pathogenicity; and d) mechanisms by which resistance can develop. Created with BioRender.com.
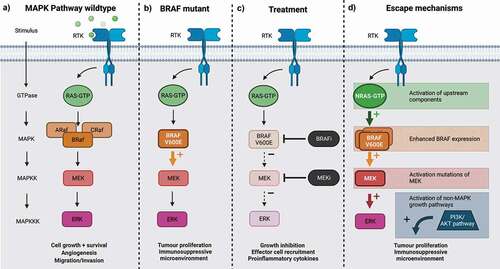
Figure 2. Potential immunological effects of BRAFi, BRAFi resistance, and mechanisms to therapeutically enhance BRAFi: a) The immunosuppressive TME created in BRAF mutant melanoma includes the recruitment and expansion of immunosuppressive cells, effector cells with reduced cancer killing potency and the secretion of factors by immune cells that can increase cancer growth and angiogenesis. b) The proinflammatory TME created by BRAFi includes increased effector immune cell recruitment and expansion, reduced immunosuppressive immune cell recruitment, and increased ability of immune effector cells to trigger cancer killing. c) As BRAFi resistance develops, the reversal of BRAFi immune effects is observed. d) There are multiple mechanisms by which BRAFi resistance can be overcome using current and future therapies. These include depleting immunosuppressive cells such as tumor-associated macrophages (TAMs), targeting angiogenesis and growth promoting vascular endothelial growth factor (VEGF), targeting immune exhaustion molecules with checkpoint inhibitors (PD-1/PD-L1), and enhancing cancer killing with adoptive cell therapy (ACT), including the use of chimeric antigen receptor T cell therapies (CAR-T), as well as stimulating effector cells, for example, by using toll-like receptor agonists (TLR). Created with BioRender.com.
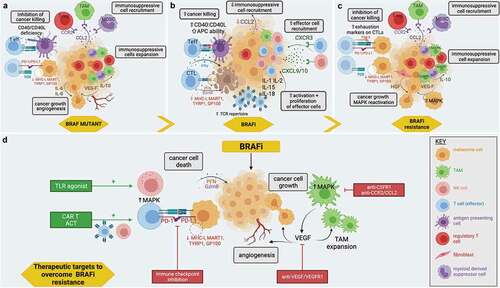
Table 1. BRAFi effect on immune function: preclinical data of the effect of BRAFi on the tumor microenvironment, specifically changes in immune mechanisms and pathways. TILS – tumor infiltrating lymphocytes; NK cells – natural killer cells; T-regs – regulatory T cells; MDSCs – myeloid-derived suppressor cells; APCs- antigen-presenting cells
Table 2. Clinical trials of combination therapies in metastatic melanoma with BRAFi/MEKi with another therapeutic intervention
