Figures & data
Figure 1. Key biologic therapies in the immunopathogenic network of myasthenia gravis, targeting B cells (*), complement (**), and FcRn (***). The AChR, presented via APC’s to CD4 + T cells via co-stimulatory molecules lead to upregulation of cytokines that stimulate B cells to produce IgG anti-AChR antibodies. The AChR IgG by fixing complement at the end-plate region, leads to destruction of the AChR’s. treg and Th17+ cells, cytokines such as IL-6 that induce tregs, and proinflammatory cytokines, such as IL-17A, IL-21, and IL-22, sustain the immune imbalance. Promising biologic therapies are currently targeting B cells (*); complement (**), activated by the antibodies to cause destruction of AChR at the postsynaptic region via MAC formation; and FcRn (***), leading to increased catabolism of IgG-AChR antibodies reducing their pathogenic effects (adapted from Dalakas MC [Citation1–3].
![Figure 1. Key biologic therapies in the immunopathogenic network of myasthenia gravis, targeting B cells (*), complement (**), and FcRn (***). The AChR, presented via APC’s to CD4 + T cells via co-stimulatory molecules lead to upregulation of cytokines that stimulate B cells to produce IgG anti-AChR antibodies. The AChR IgG by fixing complement at the end-plate region, leads to destruction of the AChR’s. treg and Th17+ cells, cytokines such as IL-6 that induce tregs, and proinflammatory cytokines, such as IL-17A, IL-21, and IL-22, sustain the immune imbalance. Promising biologic therapies are currently targeting B cells (*); complement (**), activated by the antibodies to cause destruction of AChR at the postsynaptic region via MAC formation; and FcRn (***), leading to increased catabolism of IgG-AChR antibodies reducing their pathogenic effects (adapted from Dalakas MC [Citation1–3].](/cms/asset/ef4ce446-86fb-4c89-8886-1a1d73b30d2d/ierm_a_2082946_f0001_oc.jpg)
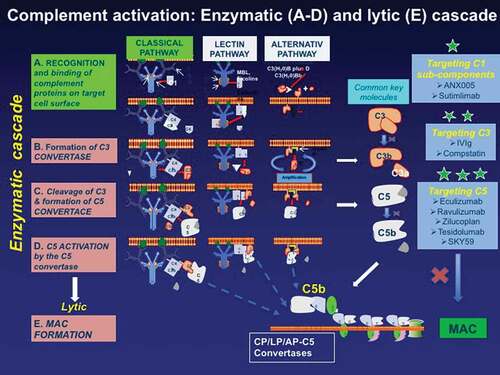
Figure 2. The main proteins involved in the complement activation cascade and the targeted complement therapeutics (highlighted with *). The complement begins with the enzymatic cascade that involves the assembly of enzyme complexes known as convertases. the enzyme cascade proceeds via three different activation pathways: the classical, the lectin, and the alternative pathway. the classical pathway begins with antibody-mediated activation of C1 that leads to the formation of the C4bC2a complex, which is the C3 convertase; the C3 convertase subsequently cleaves C3 to produce C3b, C4b C2a leading to C5 convertase complex that produces C5a and C5b. the lectin pathway begins with signal recognition by oligomeric structures which mediate the production of C4b proceeding thereafter as the classical pathway. In the alternative pathway, the C3 interacts through an amplification loop to form additional C3b that bind to C3-convertase forming a C5 convertase that cleaves C5. All three pathways generate C5b, which initiates the lytic pathway and the formation of membrane attack complex (MAC). The processes that represent therapeutic targets are shown on the the three boxes on the right along with drugs currently available or in development including: against C1* (ANX005 and Sutimilab); against C3** (IVIg and compstatin); and against C5*** (Eculizumab, Ravulizumab, Zilucoplan, Tesidolumab, and SKY59).