Figures & data
Figure 1. Pathogenic mechanisms involved in autoimmune hemolytic anemia (AIHA). Several predisposing conditions are involved in AIHA development including genetic background, presence of underlying immunodeficiencies, infections, solid and hematologic neoplasms. The main immune mechanisms responsible for tolerance breakdown are molecular mimicry, emergence of forbidden clones, and polyclonal B-cell activation. Additionally, drugs and hematologic treatments, such as checkpoint inhibitors (CPI) and hematopoietic stem cell transplant (HSCT) may induce immune dysregulation or generation of neoantigens. The resulting immunologic activation involves antigen presenting cells (APC), T- and B-cells, and cytokines and leads to the production of autoantibodies of several classes. B-cells interact with T helper (Th) cells via CD40 and its ligands but also present cognate antigens, acting as professional APC. This interaction also contributes to generate class-switched autoantibodies. Concerning cytokines, interleukin (IL)-4, IL-6, and IL-10 levels are increased and interferon gamma (IFN-γ) decreased favoring Th2 response and boosting humoral autoimmunity; the IL-2 and IL-12 levels are also increased contributing to Th1 differentiation and cellular autoimmunity. Transforming growth factor beta (TGF-β) is elevated favoring Th17 differentiation and production of IL-17 that decrease T-regulatory (Treg) levels. Once produced, IgG autoantibodies may weakly activate the complement cascade, and mainly destroy erythrocytes via antibody dependent cellular cytotoxicity and phagocytosis primarily in the spleen. IgM autoantibodies strongly activate complement system and cause erythrocyte destruction via C3b opsonization and phagocytosis primarily in the liver. These mechanisms are both called extravascular hemolysis. IgM may lead to terminal complement activation until the formation of the membrane attack complex (MAC) with consequent intravascular hemolysis.
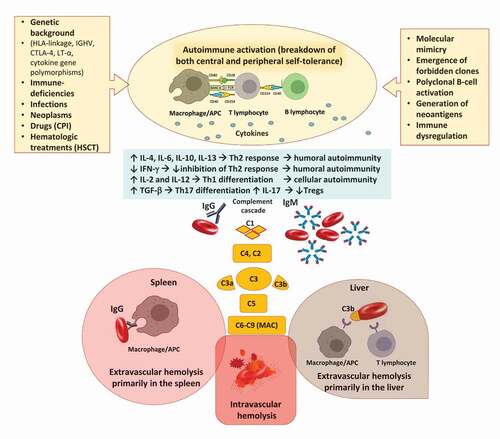
Table 1. Standard therapies for warm AIHA (wAIHA) and cold agglutinin disease (CAD).
Table 2. New drugs in warm AIHA (wAIHA) and cold agglutinin disease (CAD).
