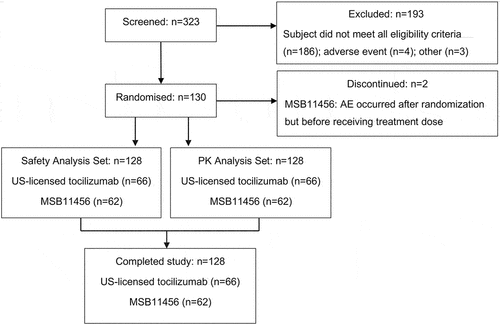
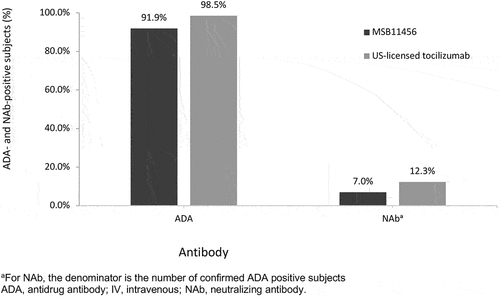
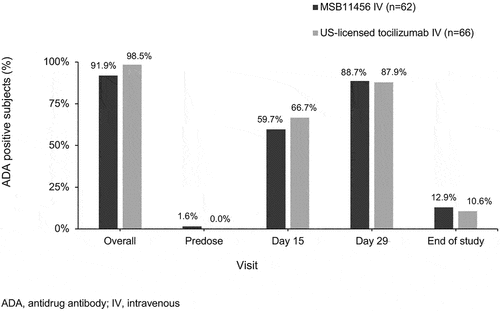
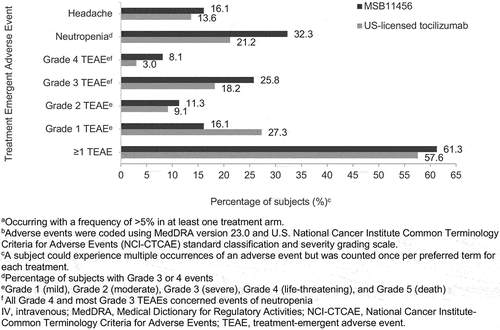
Figures & data
Table 1. Subject demographics and characteristics at baseline by treatment groups. Safety analysis set.
Figure 2. Arithmetic mean (±SD) tocilizumab serum concentrationsa versus time on a linear scale following a one-hour 8 mg/kg IV infusion of MSB11456 and US-licensed tocilizumab in healthy subjects (pharmacokinetic analysis set).
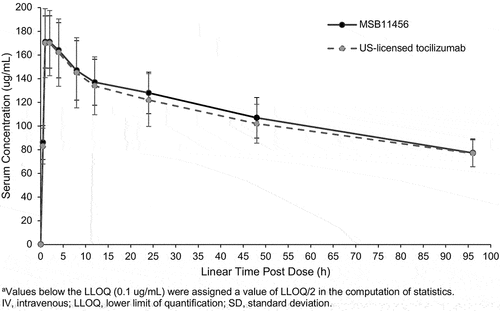
Table 2. Summary of tocilizumab serum pharmacokinetic parameters following a one-hour 8 mg/kg IV infusion of MSB11456 and US-licensed tocilizumab in healthy subjects (pharmacokinetic analysis set).
Table 3. Statistical analysis of the bioequivalence of a one-hour 8 mg/kg IV infusion of MSB11456 to US-licensed tocilizumab in healthy subjects (pharmacokinetic analysis set); primary and secondary pharmacokinetic parameters.