Figures & data
Figure 1. Overview of mechanism of action with the peanut patch.
The peanut patch contains 250 µg of dried peanut protein on a film disc that is placed on top of a doubled-sided adhesive foam ring. When placed on the skin the film disc and foam ring, which are held in place by an adhesive overlay, form a condensation chamber. Natural water loss from the skin leads to solubilization of the peanut allergen which is taken up by antigen-presenting cells, such as Langerhans cells, in the superficial layers of the non-vascularized epidermis.
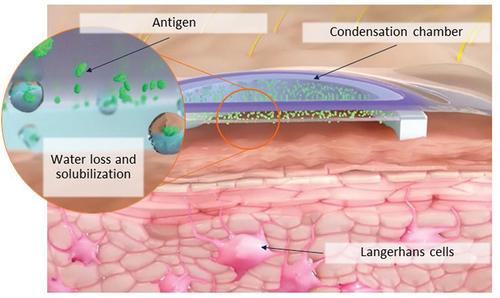
Table 1. Completed peanut patch phase 3 trials.
Figure 2. Proportion of treatment responders between active and placebo groups in children aged 1–3 years (a) and aged 4–11 years (b, c).
The primary measure of treatment effect compared the percentages of treatment responders between the active and placebo groups after 12 months of treatment among all randomized participants aged 1–3 years (a) and 4–11 years (b). Treatment responder defined as Month 12 ED ≥ 1000 mg (if baseline ED >10 mg) or ≥ 300 mg (if baseline ED ≤ 10 mg).
Proportion of participants aged 4–11 years meeting prespecified primary outcome at Months 12 and 36 in PEOPLE PP set according to primary outcome criteria in PEPITES. The PP set was defined as those participants who completed all treatment according to the study protocol without major deviations that could affect the assessment of the treatment effect, which included an evaluable DBPCFC performed as required by the protocol at Months 12 and 36 and compliance of ≥ 80% (c). CI, confidence interval; DBPCFC, double-blind, placebo-controlled food challenge; ED, eliciting dose; PP, per protocol.
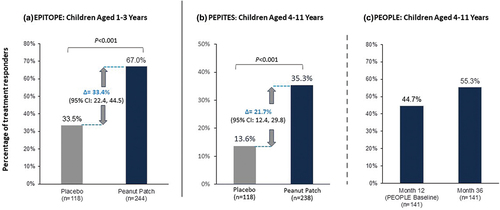
Figure 3. Proportion of participants achieving an eliciting dose of ≥ 1000 mg at month 12 with the peanut patch.
A key efficacy endpoint measured the proportion of participants achieving an ED ≥ 1000 mg after 12 months of active treatment in children aged 1–3 years in EPITOPE (a) and 4–11 years in PEPITES (b). The change in the proportion of participants aged 4–11 years achieving an ED ≥ 1000 mg between Month 12 and Month 36 was assessed in the open-label extension PEOPLE study. CI, confidence interval; ED, eliciting dose.
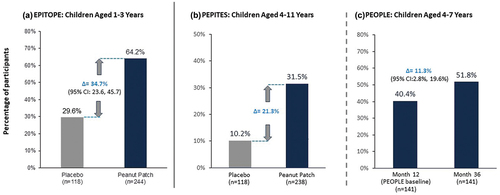
Figure 4. Changes in reaction severity during DBPCFC between month 0 and month 12.
The severity of reactions during DBPCFC was graded by the investigator according to PRACTALL scoring as absent, mild, moderate, or severe in children aged 1–3 years in EPITOPE (a) and aged 4–11 years in PEPITES (b) [Citation52].
Reaction definitions: Grade 0: negative; Grade 1: only erythema, or erythema + infiltration; Grade 2: erythema, few papules; Grade 3: erythema, many or spreading papules; Grade 4: erythema, vesicles.
DBPCFC, double-blind, placebo-controlled food challenge.
![Figure 4. Changes in reaction severity during DBPCFC between month 0 and month 12.The severity of reactions during DBPCFC was graded by the investigator according to PRACTALL scoring as absent, mild, moderate, or severe in children aged 1–3 years in EPITOPE (a) and aged 4–11 years in PEPITES (b) [Citation52].Reaction definitions: Grade 0: negative; Grade 1: only erythema, or erythema + infiltration; Grade 2: erythema, few papules; Grade 3: erythema, many or spreading papules; Grade 4: erythema, vesicles.DBPCFC, double-blind, placebo-controlled food challenge.](/cms/asset/9e8a6061-394f-49ea-bd74-21631f4175a3/ierm_a_2315221_f0004_oc.jpg)
Figure 5. Changes in frequency and severity of Treatment-emergent Adverse Events Over Time in the PEOPLE (a) and REALISE (b) trials.
Treatment-relatedness and severity were determined by the investigator.
TEAE, treatment-emergent adverse event.
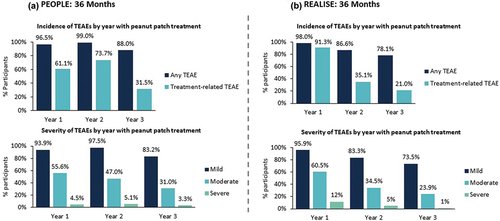
Table 2. Treatment-related adverse events in participants treated with the peanut patch: overview of the peanut patch safety profile in completed phase 3 studies.

