Figures & data
Table 1. Comparison of different 3D structural models for cancer research [30, 42, 65, 79, 80].
Table 2. Comparison of spheroid forming techniques [Citation38,Citation40–49,Citation52,Citation62,Citation69,Citation95–100].
Figure 1. Different cell culture techniques to form spheroids from cancer cells. (a) Hanging drop: gravitational pull facilitates spheroid formation (b) suspension culture: plate surface modification prevents attachment and facilitates cell aggregation (c) high viscosity suspension: media additives (e.g. methyl cellulose) increase viscosity and facilitate cell aggregation (d) pellet culture: centrifugation assists spheroid formation (e) magnetic levitation: magnetized cells aggregates to form spheroid assisted by a magnetic field (f) spinner flask: continuous agitation prevents cell attachment and facilitate spheroid formation (g) microwell plate: micropatterned wells allow uniformly sized spheroid formation (h) hydrogel embedding: hydrogel containing extracellular matrix components (laminin, collagen, etc.) aid in spheroid formation (i) fibrous scaffold: decellularized or synthetic fibrous scaffold support spheroid formation in-vitro (j) bioprinting: cells within bio ink layered in computer-aided patterns supports the formation of 3d structures (k) tumor-on-a-chip: specially designed chips containing microwells and channels supports spheroid growth with dynamically controlled environment. The illustration is created with BioRender.com.
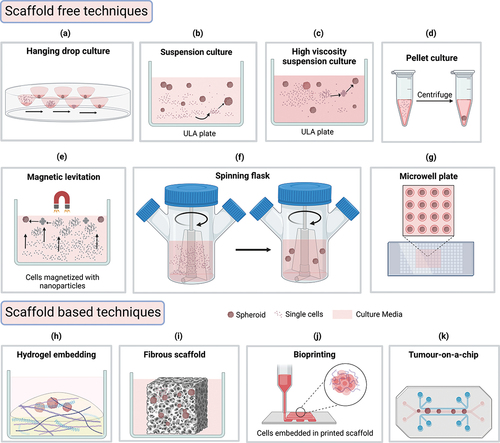
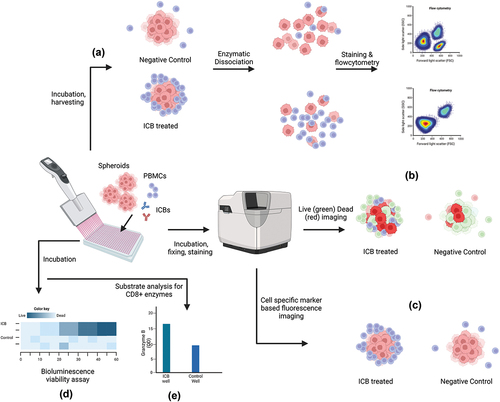
Figure 2. Multiple methods of ICB efficacy assessment from spheroid-PBMC co-culture. (a) Cocultured spheroids are harvested and dissociated for flow cytometric analysis of T-Cells to compare activated T-cell population and tumor cell population levels (b) spheroids are stained with live-dead fluorescent staining and imaged to compare the proportions of dead cells between treatment and control (c) co-cultured spheroids are fixed and fluorescently labeled for imaging analysis where the spatial distribution of immune cells is compared (d) co-cultured spheroids are treated with ATP based bioluminescence assay for viability analysis (e) co-cultured well substrates are analyzed by ELISA method to compare levels of CD8+ activation enzymes e.g. Granzyme B. The illustration is created with BioRender.com.