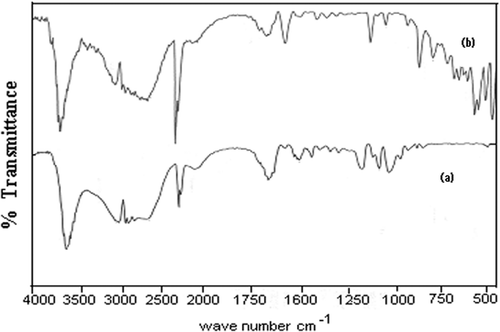
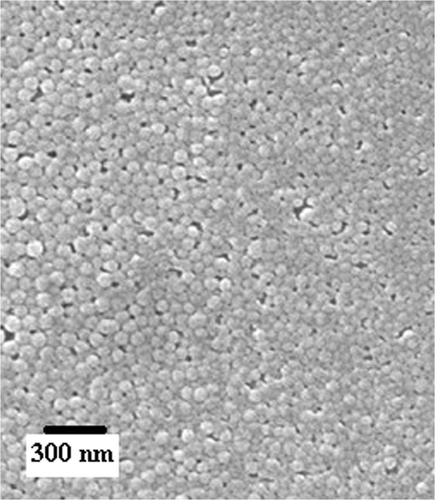
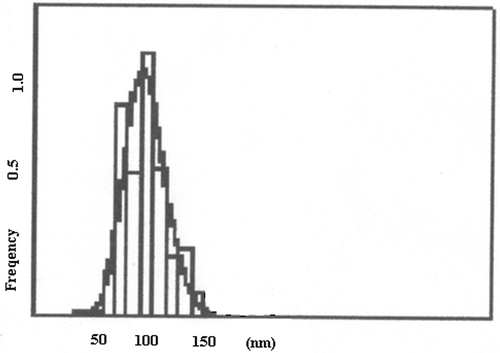
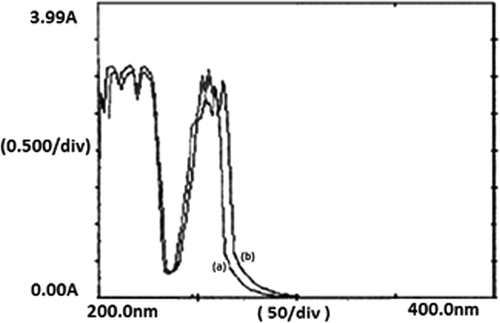
Figures & data
Table 1. Surface potential of unloaded and loaded alginate nanoparticles.
Table 2. Data showing the release exponent and diffusion coefficient under varying experimental conditions.
Figure 4. Effect of % loading of insulin on its release profile for a definite composition of nanoparticles (alginate) = 1.0 g, (calcium chloride) = 0.5 mM, pH = 7.4 and temperature = 37°C.
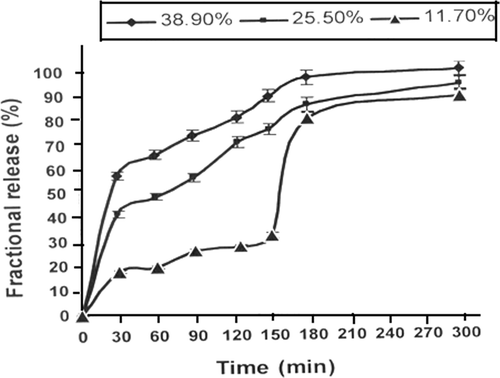
Figure 5. Effect of varying amount of alginate content in nanoparticles on release profiles of insulin for a definite composition of nanoparticles (alginate) = 1.0 g, (calcium chloride) = 0.5 mM, pH = 7.4, temperature = 37°C and % loading = 38.90%.
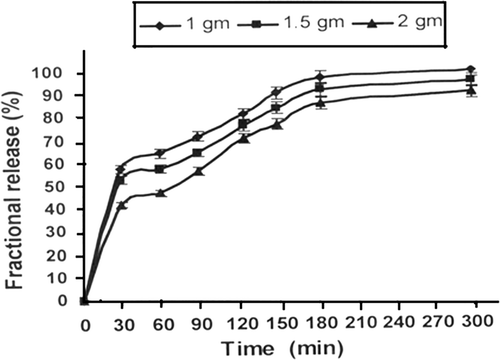
Table 3. Data showing the influence of composition and experimental condition on the equilibrium swelling ratio.
Figure 6. Effect of varying amounts of calcium chloride (crosslinker) on release profiles of insulin for a definite composition of nanoparticles (gelatine) = 1.0 g, pH = 7.4, temperature = 37°C and % loading = 38.90%.
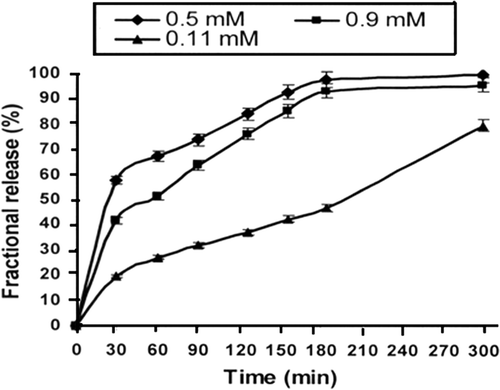
Figure 7. Variation in released amount of insulin with changing pH of the release medium for a definite composition of nanoparticles (alginate) = 1.0 g, (calcium chloride) = 0.5 mM, temperature = 37°C and % loading = 38.90%.
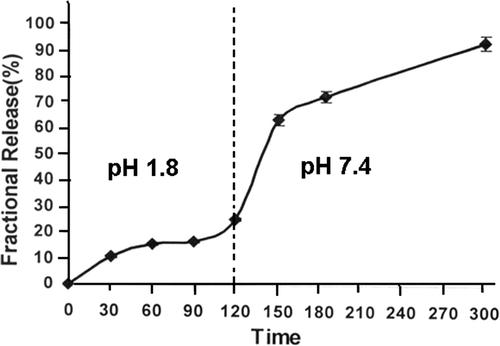
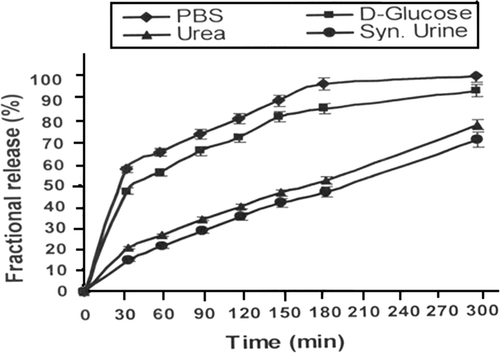
Figure 8. Effect of physiological fluids on the released amount of insulin for a definite nanoparticles composition (alginate) = 1.0 g, (calcium chloride) = 0.5 mM, pH = 7.4 and % loading = 38.90%.