Figures & data
Figure 1. SEM micrographs taken for untreated (a) and ozone-treated N-MWCNT films (b, c, d). The SEM images were obtained with an accelerating voltage of 20 kV and magnification factors of 1600× (a), 500 × (b), 2000× (c) and 6000× (d).
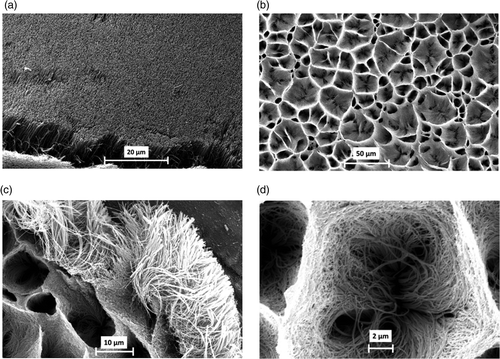
Figure 2. Raman spectra recorded for either untreated (a) or ozone-treated N-MWCNT film for 60 s (b) and 180 s (c). The symbols D and G represent the Raman D- and G-bands, respectively.
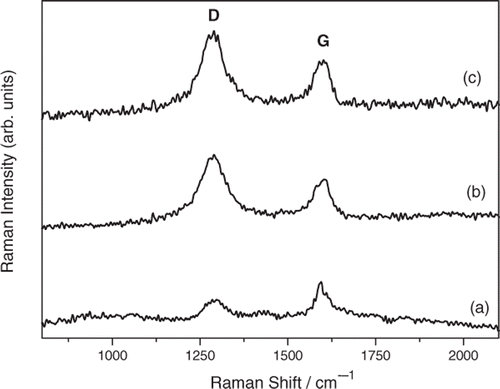
Figure 3. Variation of the intensity ratio of G- and D-bands (I G/I D) with the ozonolysis time of N-MWCNT film.
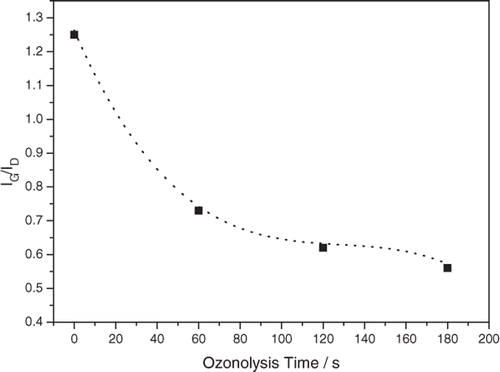
Figure 4. Representative CVs displaying the effect of ozonolysis time on electrochemical performance of N-MWCNT film. The CVs were recorded at v = 0.05 V s−1 in the presence of [Fe(CN)6]3−/4− (1.0 × 10−3 mol L−1) in KCl (0.1 mol L−1) on either untreated (dotted line) or ozone-treated N-MWCNT film for 60 s (dashed line), 120 s (dotted-dashed line) and 180 s (solid line).
![Figure 4. Representative CVs displaying the effect of ozonolysis time on electrochemical performance of N-MWCNT film. The CVs were recorded at v = 0.05 V s−1 in the presence of [Fe(CN)6]3−/4− (1.0 × 10−3 mol L−1) in KCl (0.1 mol L−1) on either untreated (dotted line) or ozone-treated N-MWCNT film for 60 s (dashed line), 120 s (dotted-dashed line) and 180 s (solid line).](/cms/asset/7b477ad5-3222-41c2-a85f-c8216d1d08af/tjen_a_664792_o_f0004g.gif)
Figure 5. Representative EIS spectra illustrating the effect of ozonolysis time on impedance behaviour of N-MWCNT film. The EIS spectra were recorded in the presence of [Fe(CN)6]3−/4− (1.0 × 10−3 mol L−1) in KCl (0.1 mol L−1) either on untreated (filled square) or ozone-treated N-MWCNT film for 60 s (filled circle), 120 s (filled upward triangle) and 180 s (filled downward triangle). For better readability, the solution ohmic resistance (∼10 Ω) was subtracted from the real part of the impedance data. The EIS spectra were recorded at the half-wave potential of [Fe(CN)6]3−/4− (E 1/2 ≈ +0.21 V versus Ag/AgCl).
![Figure 5. Representative EIS spectra illustrating the effect of ozonolysis time on impedance behaviour of N-MWCNT film. The EIS spectra were recorded in the presence of [Fe(CN)6]3−/4− (1.0 × 10−3 mol L−1) in KCl (0.1 mol L−1) either on untreated (filled square) or ozone-treated N-MWCNT film for 60 s (filled circle), 120 s (filled upward triangle) and 180 s (filled downward triangle). For better readability, the solution ohmic resistance (∼10 Ω) was subtracted from the real part of the impedance data. The EIS spectra were recorded at the half-wave potential of [Fe(CN)6]3−/4− (E 1/2 ≈ +0.21 V versus Ag/AgCl).](/cms/asset/878f3025-f298-4a45-9151-75f26b810e20/tjen_a_664792_o_f0005g.gif)
