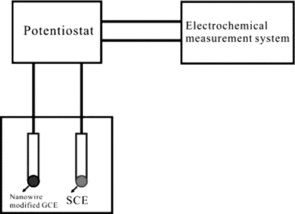
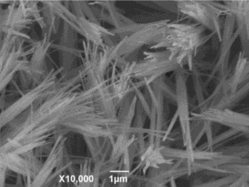
Figures & data
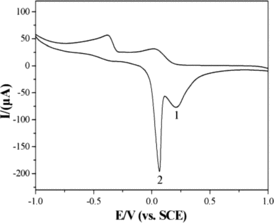
Figure 3. CVs of the bare GCE in 0.1 M KCl solution in absence (a) and presence (b) of 2 mM glyoxalic acid. CVs of the CuGeO3 nanowire modified GCE in 0.1 M KCl solution in absence (c) and presence (d) of 2 mM glyoxalic acid, scan rate, 50 mVs−1.
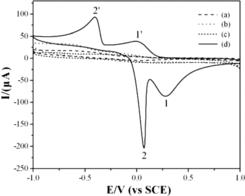
Figure 4. CVs of the CuGeO3 nanowire modified GCE in the mixed solution of 2 mM glyoxalic acid and different electrolytes. Scan rate, 50 mVs−1. (a) KBr, (b) NaOH, (c) H2SO4, (d) CH3COONa-CH3COOH.
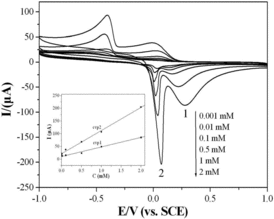
Figure 5. CVs of the CuGeO3 nanowire modified GCE in the mixed solution of 0.1 M KCl and 2 mM glyoxalic acid using different scan rates. The inset in the bottom-left part is the calibration plots of the intensities of anodic peaks against the scan rate.
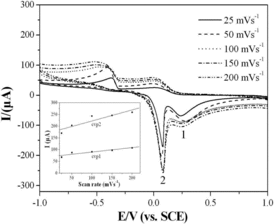
Table 1. Analytical data of the glyoxalic acid.