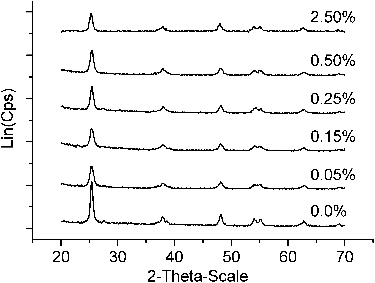
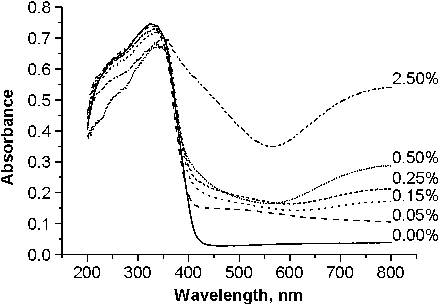
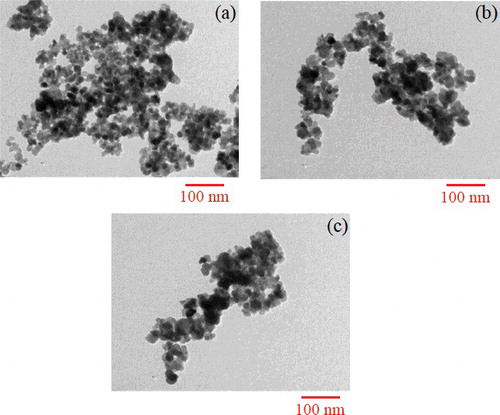
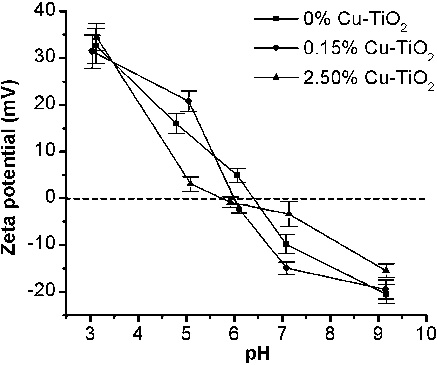
Figures & data
Table 1. BET surface area of selected Cu-doped TiO2 catalysts.
Table 2. Adsorption amount of methyl orange and methylene blue by using various Cu-doped TiO2.
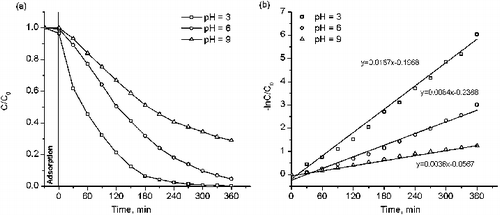
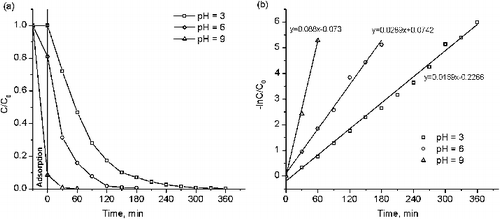
Table 3. Rate constant of the decolourisation reaction of methyl orange and methylene blue by using various Cu-doped TiO2.