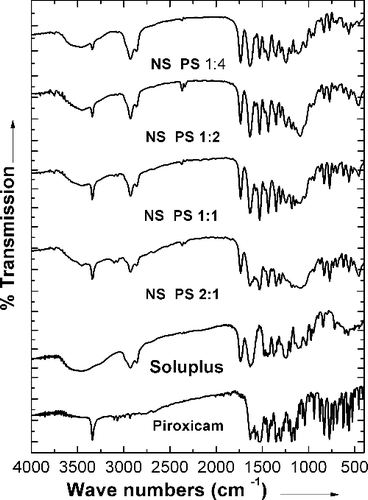
Figures & data
Table 1. Mathematical models used to predict drug release mechanism.
Figure 6. FESEM images of Piroxicam Soluplus Nanoformulations of ratios 2:1 (above) and 1:1 (Below) after 6 hours of milling.
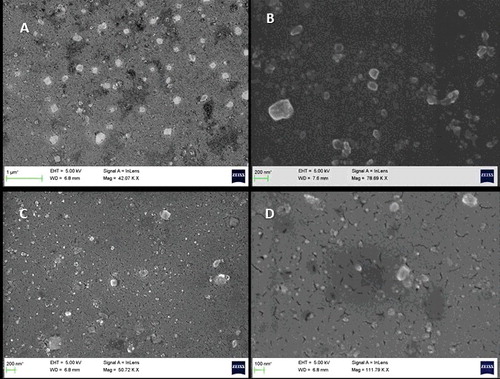
Figure 7. Caco-2 cell viability after exposure to various concentrations of Soluplus® and the nanoformulations (NS PS 1:1 and 1:4 for 12 hours). Significantly different with respect to control: *p < 0.05, #p < 0.05.
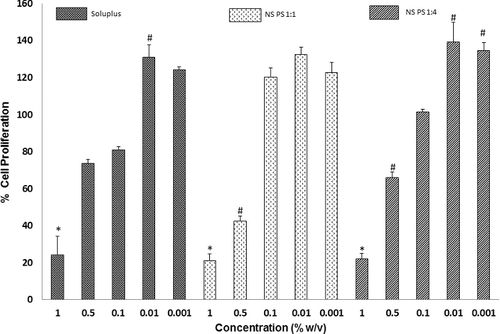
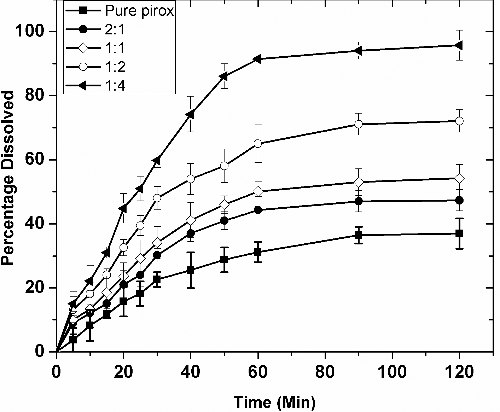
Table 2. Dissolution efficiencies achieved with varying polymer content in the formulations.
Table 3. Statistical parameters obtained after fitting the drug release data to various release kinetic models.