Figures & data
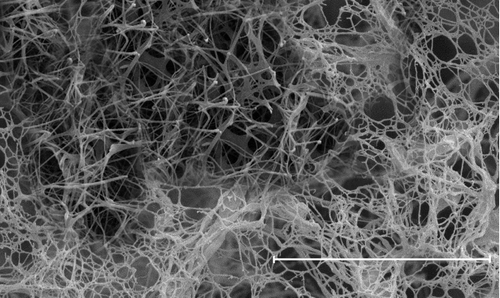
Figure 2. TEM images of the (a) as-deposited CuO on cellulose fibres, (b) sample which heated at 300 °C for 10 min, (c) the CNTNs which heated at 300 °C for 30 min. The scale bare is 100 nm.

Figure 3. Optical transmission of deposited CuO on cellulose fibres which heated at 300 °C for different times of 0, 5 and 10 min.
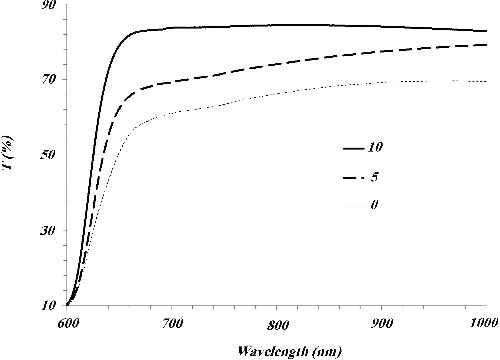
Figure 5. Experimental absorption of the CNTNs on glass substrate and calculated absorption, scattering and extinction cross sections of the CNTNs.
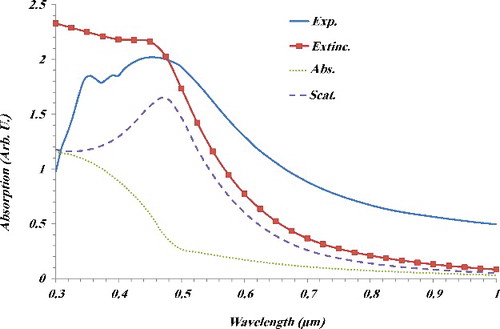
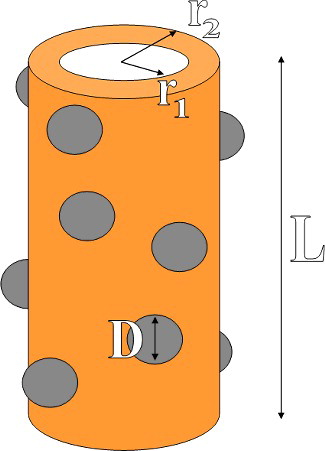
Figure 7. Schematic view of simulated CNTN. The values of the inner and outer radius of CuO nanotube are r1 = 25 nm and r2= 50 nm. Moreover, the length of nanotube and diameter of TiO2 nanoparticles are fixed at L = 2 µm and D = 20 nm.