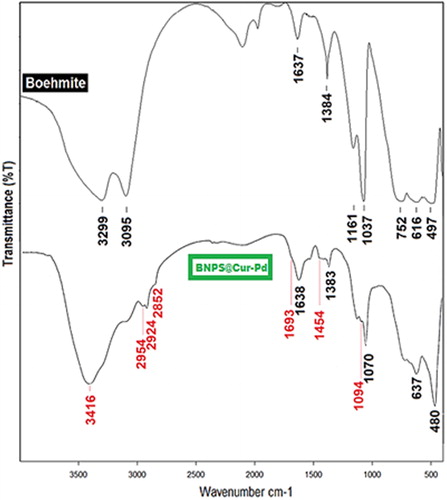
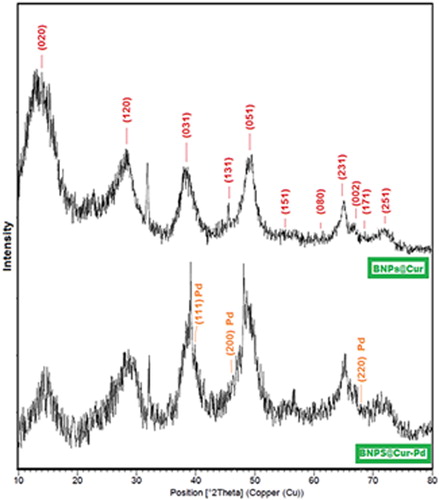
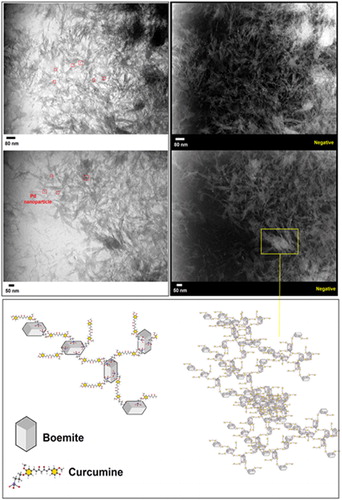
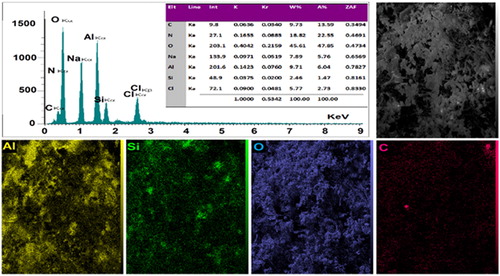
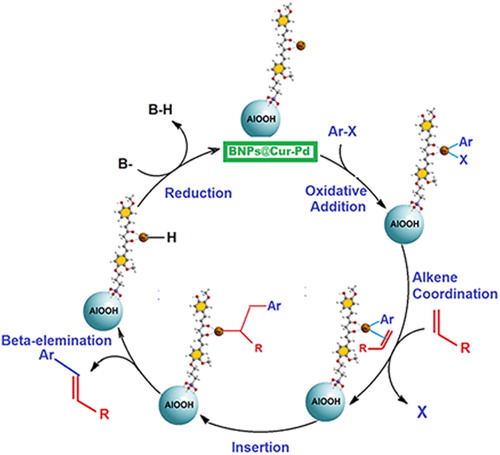
Figures & data
Table 1. Optimization of the reaction conditions for Suzuki coupling reaction in the presence of BNPs@Cur-Pd as a nanocatalyst.Table Footnotea 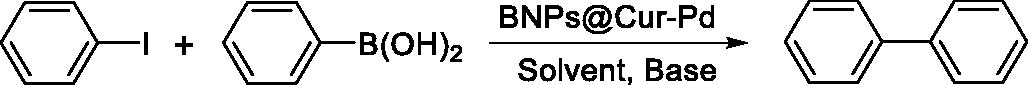
Table 2. Suzuki coupling reactions of aryl halides with aryl boronic acid catalyzed by BNPs@Cur-Pd nanocatalyst.Table Footnotea
Table 3. Optimization of the reaction conditions for Stille coupling using BNPs@Cur-Pd as a nanocatalyst.Table Footnotea
Table 4. The Stille coupling reactions of aryl halides with Ph3SnCl catalyzed by BNPs@Cur-PdTable Footnotea.
Table 5. Optimization of the reaction conditions for Heck coupling over the BNPs@Cur-Pd catalyst.Table Footnotea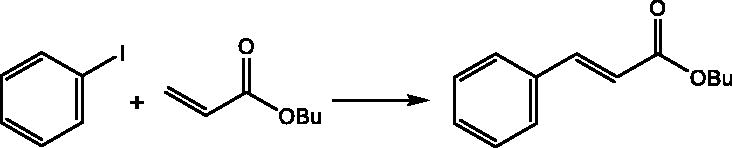
Table 6. The Heck coupling reactions for aryl halides with alkenes catalyzed by BNPs@Cur-Pd nanocatalyst.Table Footnotea
Table 7. Comparing the performance of BNPs@Cur-Pd catalyst with the previously reported procedure in the C–C coupling of iodobenzene and phenylboronic acid.