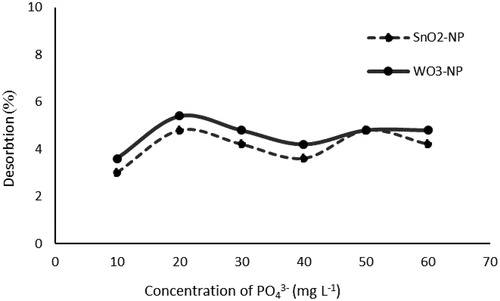
Figures & data
Figure 5. Effect of initial pH on phosphate removal (initial concentration of phosphate, 50 mg L−1;absorbent dosage, 0.025 g L−1; contact time, 24 h; Temperature, 25 °C).
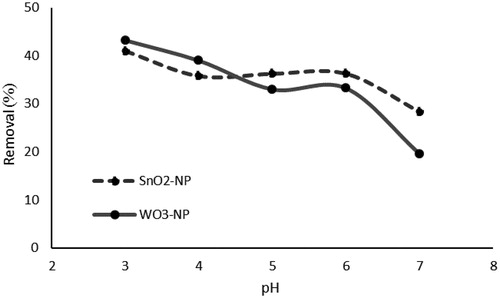
Figure 6. Effect of adsorbent dosage on phosphate removal (initial concentration of phosphate, 50 mg L−1; contact time, 24 h; Temperature, 25 °C).
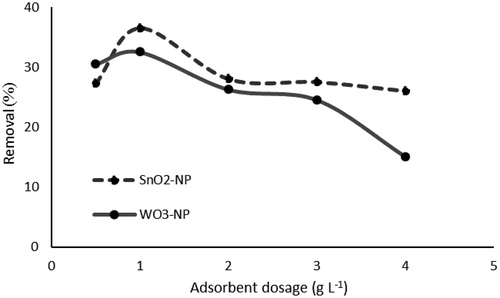
Figure 7. Effect of contact time on phosphate removal (initial concentration of phosphate, 50 mg L−1; absorbent dosage, 1g L−1; Temperature, 25 °C).
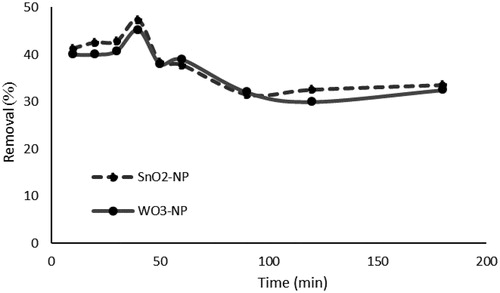
Table 1. Kinetic parameters for the adsorption of phosphate onto nano adsorbent.
Table 2. Maximum adsorption capacities of phosphorus onto various adsorbents.
Figure 8. Freundlich isotherm model of phosphate adsorption (pH, 3; adsorbent dosage, 1 g L−1; contact time, 40 min; Temperature, 15 °C).
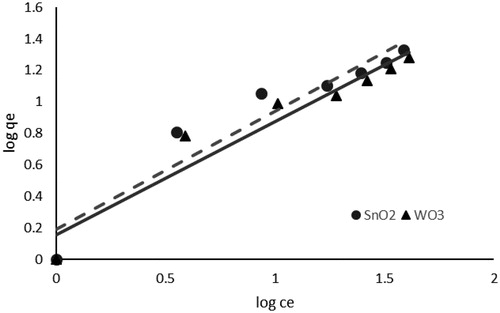
Table 3. Freundlich isotherm constants for the adsorption of phosphate onto nano adsorbent.
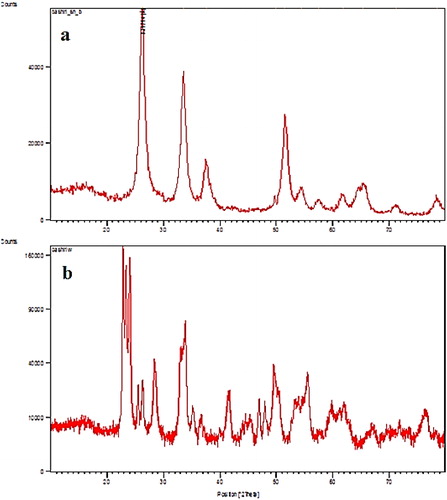
Figure 9. SEM image after adsorption isotherm of SnO2 (a) and WO3 (b) nano adsorbent (initial concentration of phosphate, 60 mg L−1).

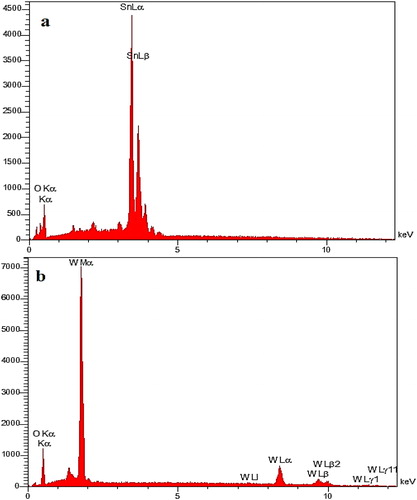
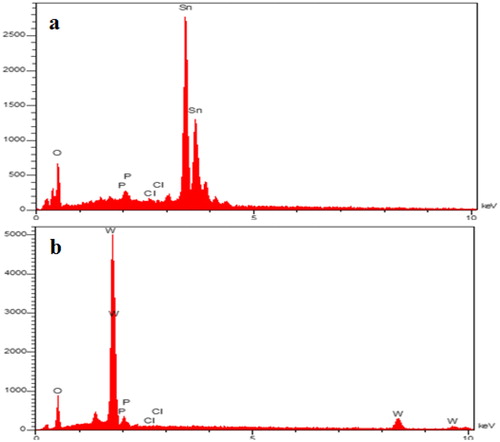
Figure 10. Energy Dispersive X-Ray (EDX) spectrum after adsorption isotherm of SnO2 (a) and WO3 (b) nano adsorbent (initial concentration of phosphate, 60 mg L−1).
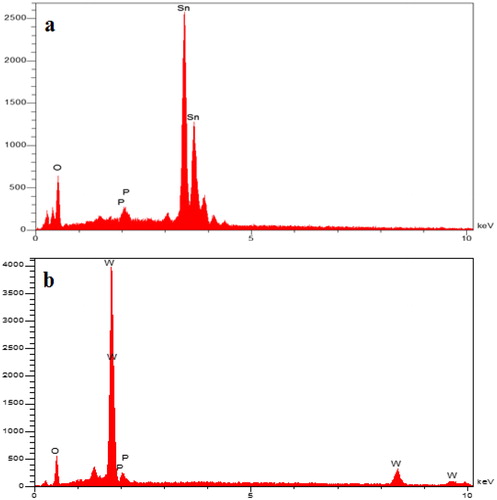
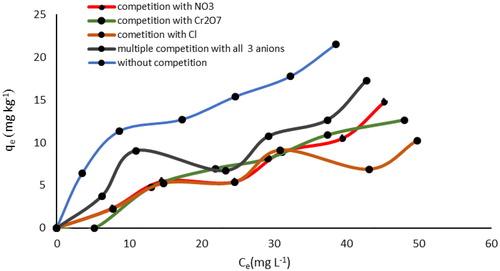
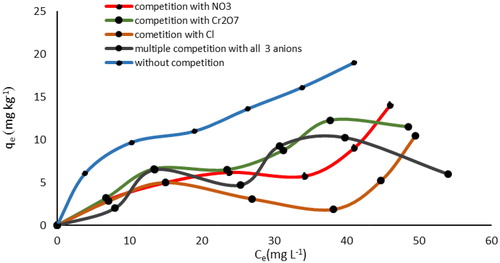
Figure 15. SEM image of SnO2 (a) and WO3 (b) nano adsorbent with coexisting Cl−, Cr2O7– and NO3− anions.

Figure 17. EDX spectrum of SnO2 (a) and WO3 (b) nano adsorbent with coexisting Cl−, Cr2O7− and NO3- anions.
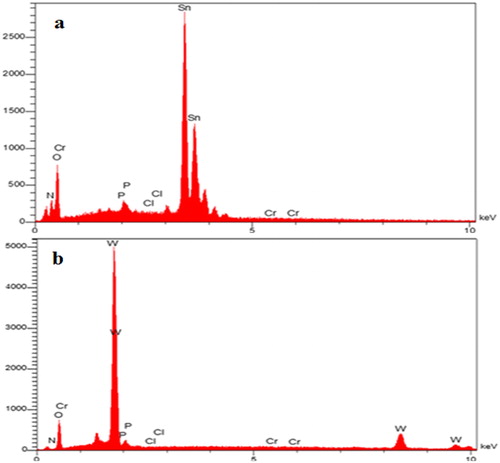
Figure 18. Effect of temperature on phosphorus removal (initial concentration of phosphate, 50 mg L−11; absorbent dosage, 1g L−1; pH, 3; time, 40 min).
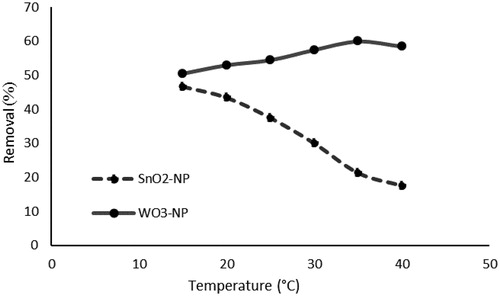
Table 4. Thermodynamic parameters for adsorption of phosphate onto nano adsorbent.