Figures & data
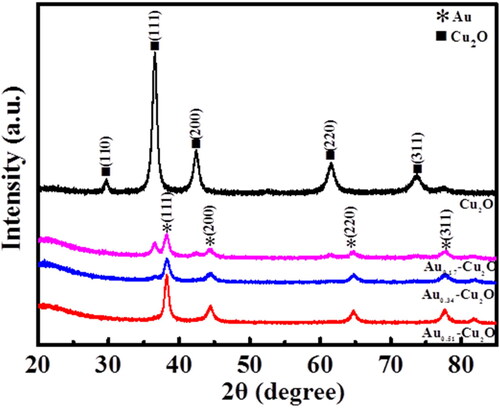
Figure 1. Schematic diagram of the formation process of Aux-Cu2O composites. (The main galvanic replacement reaction is 3Cu+ + AuCl4− → 3Cu2+ + Au + 4Cl−. The whole reaction is carried out under normal temperature and pressure).
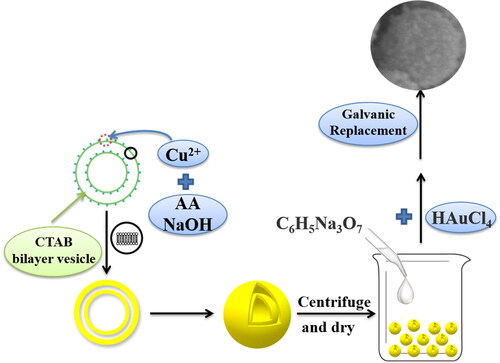
Figure 3. (a) and (b) SEM images of hollow Cu2O nanospheres; (c) and (d) HRTEM images of hollow Cu2O nanospheres.

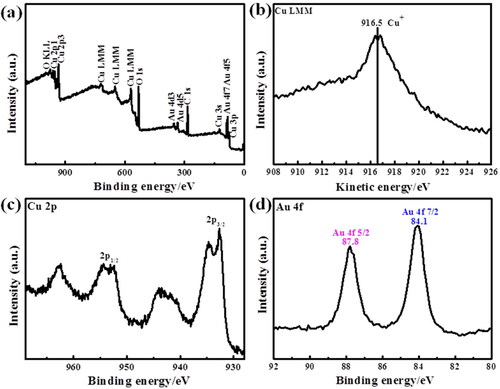
Figure 4. (a) and (b) SEM images of Au0.17-Cu2O composite; (c)–(e) HRTEM images of Au0.17-Cu2O composite; (f)–(h) Element mapping of Cu, Au and O of Au0.17-Cu2O composite.

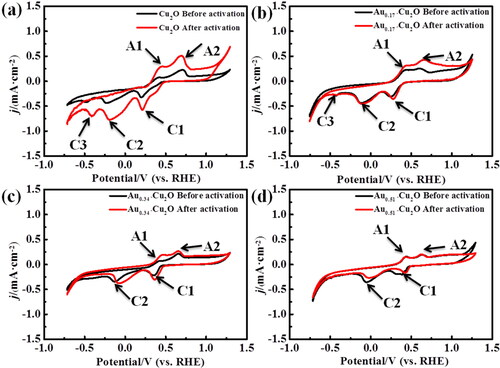
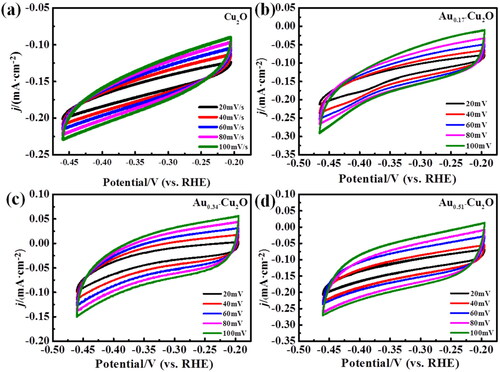
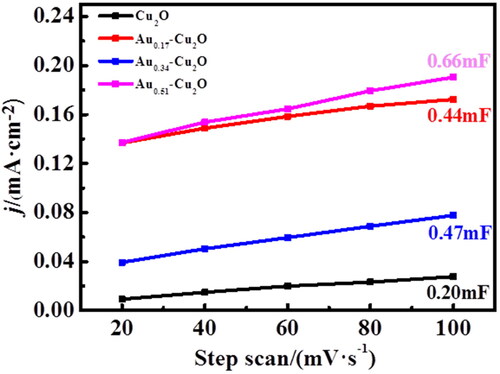
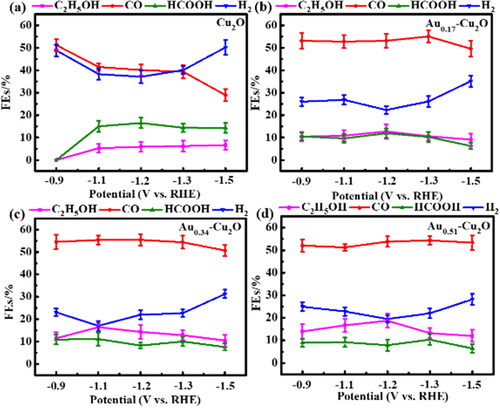
Figure 6. (a) Cu2O, (b) Au0.17-Cu2O, (c) Au0.34-Cu2O, (d) Au0.51-Cu2O in N2-saturated 0.1 M KHCO3 solution, scan rate = 10 mV/s.