Figures & data
Table 1. Formulation development design.
Figure 1. FTIR analysis of various formulations: (A) F3, (B) F13, (C) F23, (D) F8, (E) F18, (F) F28.
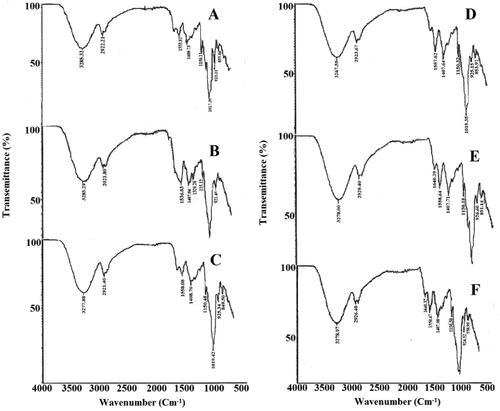
Table 2. FTIR absorption values of various formulations: (A) F3, (B) F13, (C) F23, (D) F8, (E) F18, (F) F28.
Figure 2. TGA curves of non-drug and drug-loaded formulations of non-irradiated and γ-irradiated nanocomposite films.
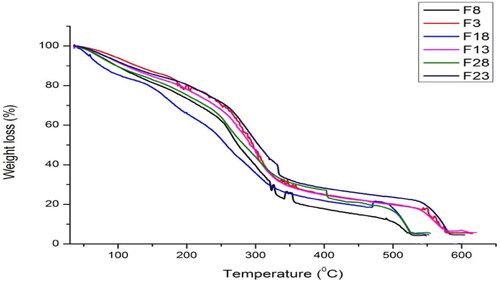
Table 3. Thermal studies of non-drug and drug-loaded formulations of non-irradiated and γ-irradiated bionanocomposite films at different temperatures.
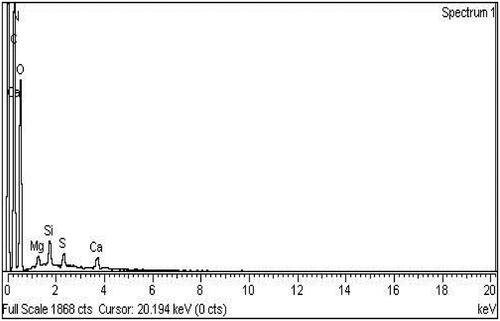
Figure 3. SEM image of cefixime loaded formulation F23 irradiated with 80 kGy dose (a) at a magnification of 2500×, (b) at a magnification of 5000×, (c) at a magnification of 10,000×, (d) non-drug loaded (F8) at magnification of 10,000×.
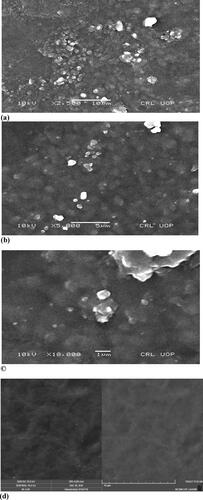
Table 4. Elemental composition of 80 kGy γ-irradiated formulation F23.
Table 5. Solubility studies of cefixime in different solvent systems at 37 ± 2 °C for 24 h.
Figure 5. Standard calibration curve of cefixime made with series of dilutions ranging from 1 µg/ml to 10 µg/ml.
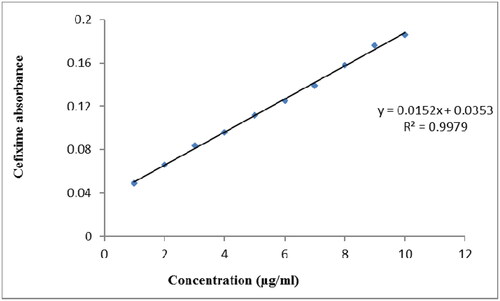
Table 6. Drug content uniformity/drug loading efficiency of formulations.
Figure 6. The swelling ratio of chitosan-starch nanocomposite films in (A) HCl buffer pH 1.2, (B) Acetate buffer pH 4.5, and (C) Phosphate buffer pH 6.8.
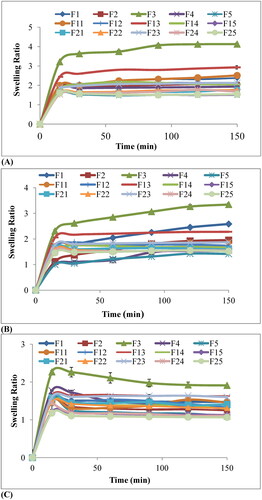
Figure 7. Percentage water content of chitosan-starch nanocomposite films in (A) HCl buffer pH 1.2, (B) Acetate buffer pH 4.5, and (C) Phosphate buffer pH 6.8.
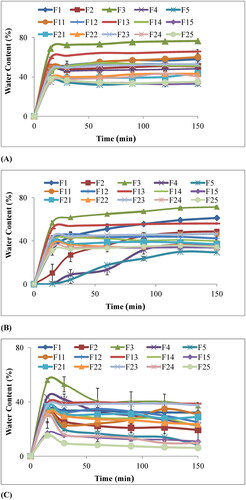
Table 7. Percentage matrix erosion of non-irradiated and γ-irradiated bionanocomposite films at pH 1.2; 4.5 and 6.8.
Figure 8. (A) In vitro permeation profile of non-irradiated cefixime nanobiocomposite films; (B) 40kGy γ-irradiated cefixime nanobiocomposite films; (C) 80 kGy γ-irradiated cefixime nanocomposite films.
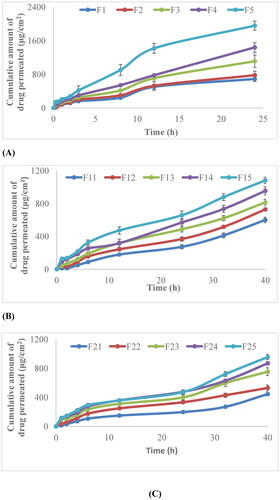
Table 8. Ex vivo skin permeation kinetic data of cefixime from both γ-irradiated and non-irradiated films.
Figure 9. (A) Release behavior of non-irradiated nanocomposite films, (B) γ-irradiated films at a dose of 40 kGy, (C) γ-irradiated films at a dose of 80 kGy.
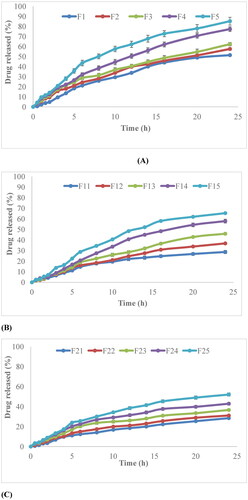
Table 9. Kinetic profile of γ-irradiated and non-irradiated bionanocomposite films.
Table 10. Comparison of similarity factor (f2) of different formulations.
Table 11. Antibacterial activities of different bionanocomposite films (γ-irradiated and non-irradiated) against different bacteria.
Data availability
All the data have been added to this article.