Figures & data
Figure 2. UV–visible absorption spectroscopy of (a) citrate- and (b) acrylate-stabilized gold nanoparticle solutions as a function of pH. The UV–visible spectra were collected 24 hours after changing the pH of the solutions and were nearly identical to the spectra recorded at its original pH.
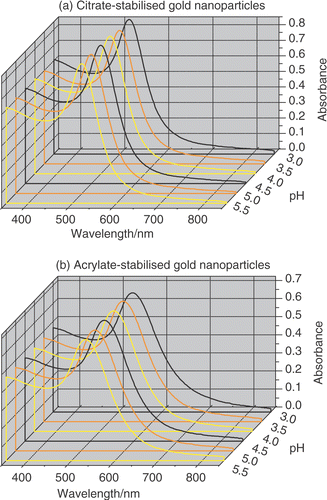
Table 1. Characterization of citrate- and acrylate-stabilized Au nanoparticles.
Figure 3. Zeta potential of (a) citrate- and (b) acrylate-stabilized gold nanoparticles measured between pH 3.0 and pH 5.5. The zeta potential was measured 10 minutes after changing the pH.
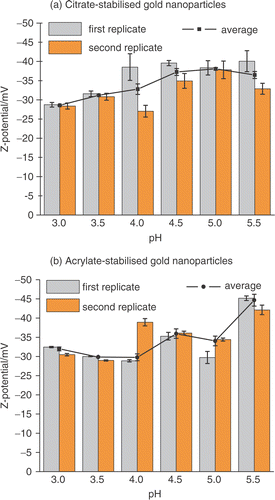
Table 2. Characterization of the surfaces studied.
Figure 4. Tapping mode AFM images (500 nm × 500 nm) of (a) clean Si/SiO2, (b) NPPTMS monolayer on Si/SiO2 and (c) APTMS monolayer on Si/SiO2.
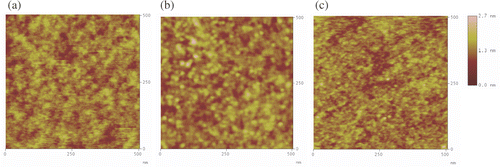
Figure 5. XPS N (1s) spectra of a monolayer of (a) APTMS and (b) NPPTMS on Si/SiO2 taken after 15 minutes of continuous exposure to X-ray irradiation.
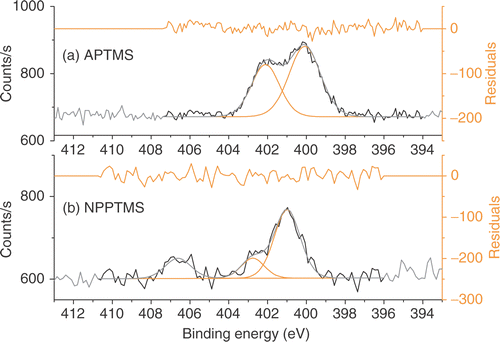
Figure 6. Tapping mode AFM images (500 nm × 500 nm) of Si/SiO2 surfaces immersed in (a) citrate- and (b) acrylate-stabilized gold nanoparticle solutions at different pHs.
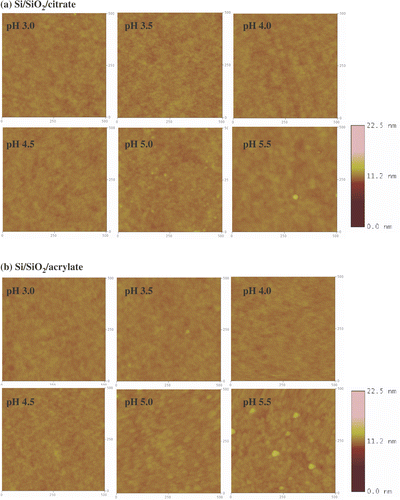
Figure 7. Tapping mode AFM images (500 nm × 500 nm) of NPPTMS monolayers immersed in (a) citrate- and (b) acrylate-stabilized gold nanoparticle solutions at different pHs. The z-scale bar for pH 3.0 is to the right of the image. The z-scale bar for pHs from 3.5 to 5.5 is to the right of the image of pH 5.5.
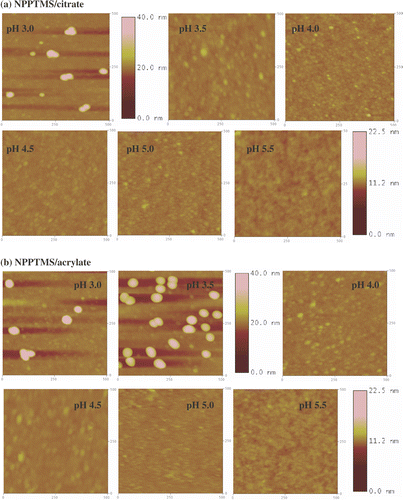
Figure 8. Tapping mode AFM images (500 nm × 500 nm) of APTMS monolayers immersed in (a) citrate- and (b) acrylate-stabilized gold nanoparticle solutions at different pHs. The z-scale bar for pH 3.0 and pH 3.5 is to the right of the image of pH 3.5. The z-scale bar for pHs from 4.0 to 5.5 is to the right of the image of pH 5.5.
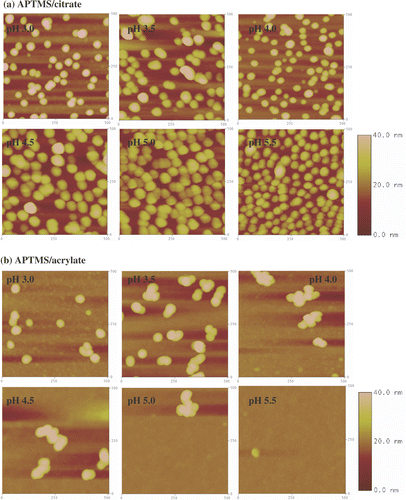
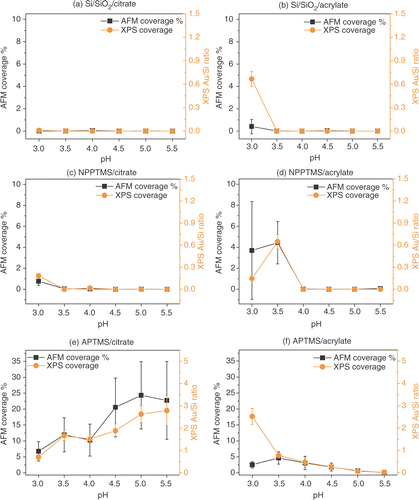
Figure 9. Surface coverage estimated by AFM and by XPS (as gold to silicon ratio) for Si/SiO2 surfaces immersed in (a) citrate- and (b) acrylate-stabilized gold nanoparticles, NPPTMS monolayers immersed in (c) citrate- and (b) acrylate-stabilized gold nanoparticles and APTMS monolayers immersed in (d) citrate and (e) acrylate-stabilized gold nanoparticle solutions, at different pHs. The XPS coverages shown above were scaled in order to allow direct comparison with the AFM coverages. This was achieved by multiplying the XPS data by an arbitrary scaling factor and calculating the residuals between the AFM and XPS coverages at each pH. The best value for the scaling factor was then found by minimizing the standard deviation of the data set. Thus, XPS data points can be directly read across to AFM percentage coverages.