Figures & data
Figure 1. Low-frequency resonance Raman spectra of sol--gel encapsulated deoxyMb. The top spectrum (a) is at pH 7.0. After the jump of pH from 7.0 to 2.6, spectra were taken at (b) 5 min, (c) 15 min, and (d) 100 min. Each spectrum shows the data collected at 30 sec time intervals for spectra (a), (b), and (c) and 15 min for spectrum (d). For better clarity, the intensity of spectrum at Raman shifts in the range of wave numbers 200–1700 cm−1 for spectra (a--d) is vertically displaced (on left) and (a--b) (on right). The intensity of spectrum (d) is normalized to the same collection time (30 sec). Modified from the Reference with permission Citation7.
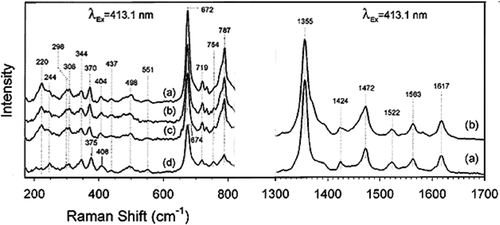
Figure 2. A detailed edge view geometry of the FeO2 bond in the myoglobin molecule in the top row. The bound dioxygen is bent, held end-on and forms a hydrogen bond to the distal (E7) histidine at position 64, with the angles of +Fe–O–O = 115° and angle between O–O–H = 96°. A simplified molecular orbital (MO) representation of the FeO2 molecule is shown at the bottom. Modified from reference with permission Citation7.
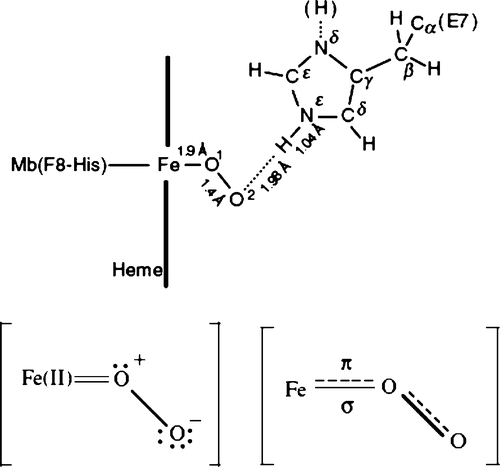
Figure 3. Schematic representation of the basal autoxidation process in water with the rate constant kH2O and the protoncatalyzed process with the rate constant kH H2O in neutral pH range. For sperm whale MbO2, kH2O = 0:18 × 10−4 h−1M−1 and = 0:20 × 104 h−1M−2 in 0.1 M buffer at 25°C. The aqua-met Mb formed by each process can be isomerized into the other. Modified from reference with permission Citation8.
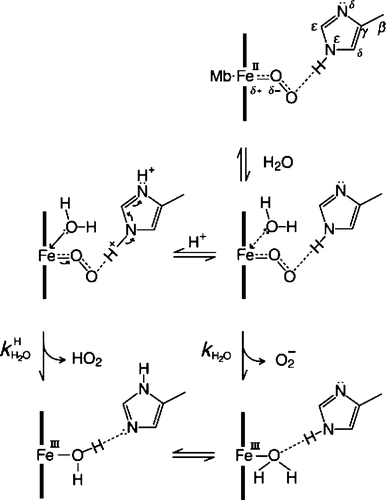
Table 1. Low-frequency resonance Raman spectra intensity (in arbitrary units or a.u.) of sol--gel encapsulated deoxyMb represented as base value (100) and reduced intensity value (in%) from base 100 a.u. at different pHs (column 1) and duration (column 2). The Raman shifts show more sensitivity at excitation frequency λex = 441 nm* (shown here) than λex = 413 nm (not shown). The reduced intensities of spectral peaks at low pH after jump and duration of time are notable due to Fe--His F8 linkage disruption and conformational fluctuations resulting in heme pocket unfolding or opening and more protonated His pocket in myoglobin. The formation and existence of intermediates are shown as Raman shifts or wave number (cm−1) in columns 3–7 represented as spectral peaks in .
Figure 4. Role of the globin moiety in stabilizing the FeO2 bond in myoglobin. Notice the globin chain folding in relation to time. Redrawn from the reference with permission Citation8.
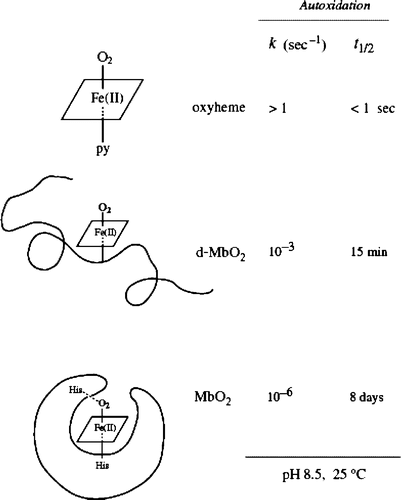
Figure 5. (On leftmost): A electron microscopic TEM image of Fe2O3; (on second left) SEM of albumin microspheres; (on third panel) TEM image of polyethylene coated microspheres; (on rightmost panel): A SEM image of polystyrene coated microsphere (shown with arrow) attached to red blood cell. Redrawn from references Citation1, Citation13.
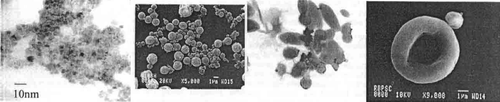
Figure 6. The figure represents the scheme of two-site immunoassay to develop magnetic immunoassay using anti-myoglobin biotinylated antibodies and tagged biomarker to measure myoglobin. Redrawn from reference Citation15.
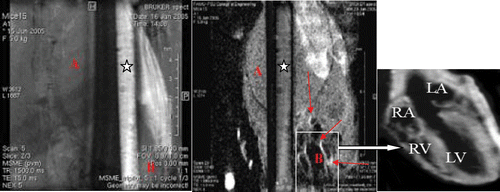
Figure 7. (On left panel) The figure represents MRI images of a capillary filled with iron oxide--myoglobin at pH 7.4 in presence of phosphate buffered saline pH7.4. In the background, mice body cross-sectional single slice image is shown with heart image (at the botton on right) without contrast agent. (On right panel) Same capillary with myoglobin at pH 4.0 in presence of acetate buffer pH 4.0 shows loss of signal of iron--oxide--myoglobin shown with asterisk. The images were generated using multislice multiecho spin echo proton density weighted technique at scan parameters TE = 15 ms, TR = 1500 ms, matrix 256 × 256, NEX = 2, FOV = 2.5 cm at 11.7 T or 500 MHz MRI imager. In the background, a same cross-sectional slice image of mice image after anti-myoglobin-iron oxide superparamagnetic contrast injection with cardiac territories are shown by arrows and details of different heart chambers (marked as RA, LA, LV, RV) can be seen in insert. Notice the enhancement of vascular wall regions rich with anti-myoglobin bound superparamagnetic particles after injection at pH 7.4 while the same imaging contrast agent in the capillary turned darker at pH 4.0.