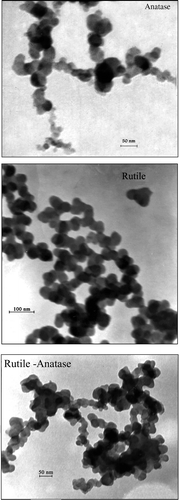
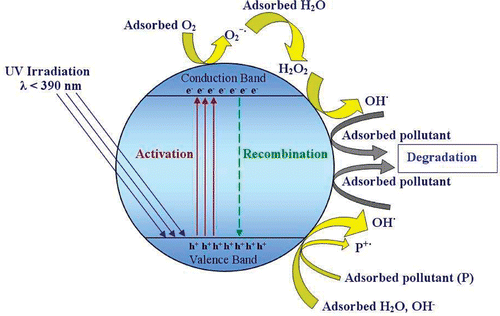
Figures & data
Table 1. Crystallographic properties of rutile and anatase Citation4.
Table 2. Structure and characteristics of C. I. Acid Blue 9.
Figure 1. Flow chart of the steps involved in the preparation of TiO2 nanoparticles of anatase (left) and rutile (right).
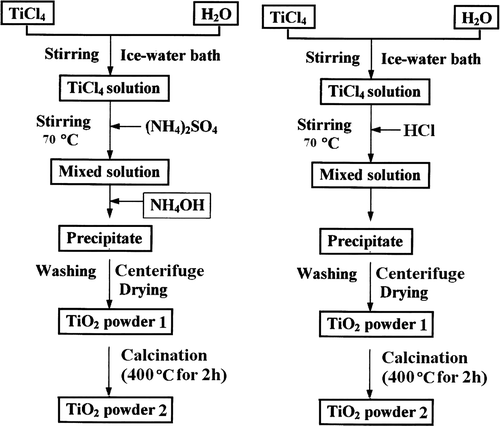
Figure 2. Proposed mechanism for preparation of TiO2 with rutile or anatase phases: (a) the orientation of the third octahedron determines whether a rutile or an anatase nucleus is formed; (b) interaction between and
octahedral hydroxyls; (c) two
octahedra share edge in the presence of
; (d) formation of anatase in the presence of
.
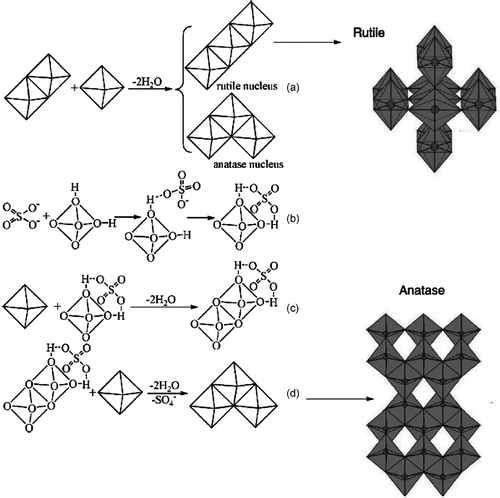
Figure 3. X-ray diffraction (XRD) patterns of synthesised TiO2 samples: (a) anatase, (b) rutile (c) rutile–anatase.
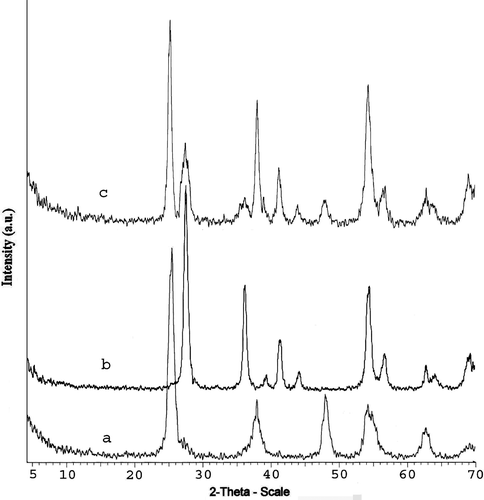
Figure 5. SEM image (left) and XRD pattern (right) of the amorphous TiO2 powder dried at room temperature under vacuum.
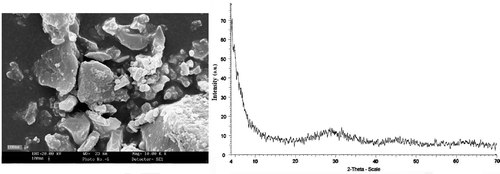
Figure 7. Comparison of the photocatalytic activity of different synthesised TiO2 nanoparticles. [TiO2]0 = 150 mg L−1, [AB9]0 = 20 mg L−1, pH = 6.2, V = 50 mL, I = 11.2 W m−2: (a) during different irradiation times, (b) at the irradiation time of 90 min.
![Figure 7. Comparison of the photocatalytic activity of different synthesised TiO2 nanoparticles. [TiO2]0 = 150 mg L−1, [AB9]0 = 20 mg L−1, pH = 6.2, V = 50 mL, I = 11.2 W m−2: (a) during different irradiation times, (b) at the irradiation time of 90 min.](/cms/asset/c9bb81c4-be33-4a25-b42e-3ee78e9f15de/tjen_a_393166_o_f0007g.gif)
Table 3. Physicochemical characteristics of the synthesised TiO2 nanoparticles.
Figure 8. Models of mixed-phase TiO2: (a) rutile islands surround anatase particles, and rutile is an electron sink; (b) a small rutile core surrounded by anatase crystallites, where electrons are shuttled from rutile to anatase.
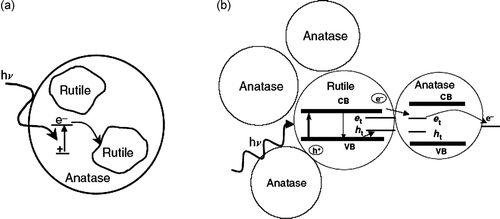
Figure 9. UV-Vis spectrum of AB9 (20 mg L−1) during photocatalysis in the presence of the synthesised mixed-phase TiO2 nanoparticles. [TiO2]0 = 150 mg L−1, pH = 6.2, I = 11.2 W m−2.
![Figure 9. UV-Vis spectrum of AB9 (20 mg L−1) during photocatalysis in the presence of the synthesised mixed-phase TiO2 nanoparticles. [TiO2]0 = 150 mg L−1, pH = 6.2, I = 11.2 W m−2.](/cms/asset/5f551818-55ea-4aa8-9974-4974895122a8/tjen_a_393166_o_f0009g.gif)