Figures & data
Figure 1. Multiple mutations can drive cancer through at least four mechanisms. From left to right: 1) Multiple mutations in different proteins along the same signaling pathway can strengthen oncogenic signals. 2) Multiple mutations in different proteins in parallel pathways. 3) Multiple mutations in different proteins in complementary pathways. 4) Multiple mutations incis, that is, on the same protein, can also activate oncogenic signaling more potently than their single driver components
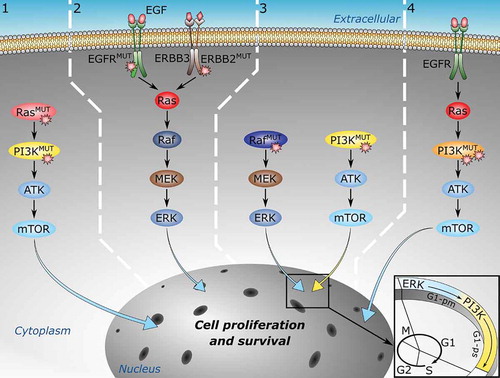
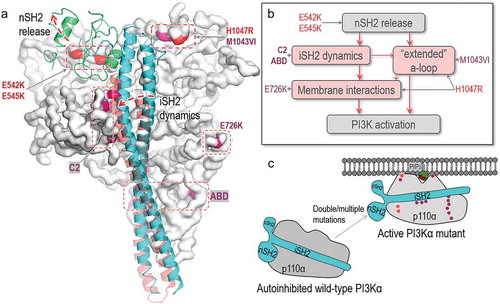
Figure 2. Structural insights into oncogenic mutations in PI3Kα. (A) PI3Kα has hotspot mutations and weak mutations. The hotspot mutations include E542K, E545K in the helical domain and H1047R in the kinase domain. The weak mutations include E726K and M1043VI in the kinase domain, N345K, C420R, E453K/Q in the C2 domain and R38H/C, R88Q, R93Q, R108H, G118D in the ABD domain. The p110α catalytic subunit is shown as the white surface. The hotspot and weak mutations are highlighted in red and pink. The iSH2 and nSH2 domains in p85α regulatory subunit are shown in cyan and green cartoon. (B) The roles of PI3Kα mutations in activation suggest that the hotspot and weak mutations induce the conformational changes for PI3Kα activation. They can be additive or cooperative. “a-loop” denotes activation loop. (C) The schematic diagram shows the activation of PI3Kα by oncogenic mutations